| Description |
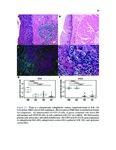
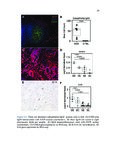
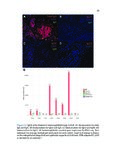
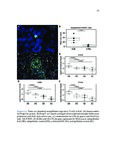
Eosinophilic esophagitis (EoE) is an immune-mediated disease of the esophagus that is commonly triggered by food antigens. EoE was uncharacterized prior to 1993, but it is now the most common cause of dysphagia (difficulty swallowing) and food impaction requiring esophageal endoscopy. EoE causes acute food impaction that requires emergency endoscopic removal. Chronic EoE causes fibrostenotic stricture formation and esophageal remodeling, and patients require periodic esophageal dilatation in order to eat. The mechanism of fibrosis in EoE is poorly studied, and optimal prevention strategies are unknown. EoE is diagnosed by endoscopic biopsy, which is invasive and incurs significant costs for patients. Food antigens that trigger EoE are determined through diet elimination trials, which can span years and consist of routine, near-monthly biopsies. This dissertation focuses on three clinical needs for EoE patients, which are the following: 1) to investigate the mechanism of fibrosis, 2) to develop a noninvasive diagnostic modality, and 3) to develop a method for rapid identification of trigger antigens. We used RNA-seq and histopathologic validation to show that a subepithelial immunoregulatory response is characteristic of EoE and can induce fibrosis. We used RNA-seq and quantitative protein assays to discover diagnostic biomarkers in esophageal secretions collected by noninvasive brushing. Finally, we developed an assay to accurately predict trigger foods using food-specific antibodies in esophageal secretions. These research methods were applied to other diseases. We performed the first characterization of eosinophilic granulomatosis with polyangiitis, a rare and fatal systemic vasculitic disease, and identified possible pathogenic mechanisms and drug targets. We are the first to perform deep-sequencing of cell-free DNA and machine learning to distinguish between hormone-sensitive and castration-resistant metastatic prostate cancer, and to identify targetable genomic alterations associated with disease progression. These demonstrate the broad utility of computational genomics for translational medicine. |