| Description |
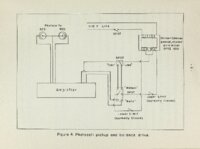
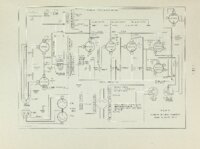
An apparatus for making rapid thermogravimetric analysis was constructed from readily assembled standard parts. A chainweight balance with optical lever was adapted so the light beam registered on either of two photocells depending upon the direction of unbalance, Current from the photocells was amplified and used to drive a small reversing motor which added or subtracted chain on the balance as required, The motor drive also turned a linear potentiometer which drove an electronic strip chart recorder. A sample of thermally reactive material was suspended from one pan of the balance into an electrically heated tube furnace. The magnitude of the weight loss (or gain) occurring in the sample during the reaction is recorded instanteously by the electronic recorder. The above apparatus was used to follow the decomposition of calcium carbonate crystals under carefully controlled temperature' and atmospheric conditions. The rate of decomposition as derived from experimental evidence and kinetic considerations is given by the following expression, where the rate is given in weight loss per unit of area per unit of time; Rate = P C02 and 02 are the measured and equilibrium pressures of CO2, ii respectively; R is the rate of weight loss in nitrogen atmosphere; B is e temperature dependent and is experimentally determined, |