| OCR Text |
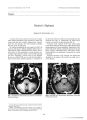
Show Journal of Neura- Ophlhalmology 17( 3): 151- 155, 1997. © 1997 Lippincoll- Raven Publishers, Philadelphia Temporal Crescent Syndrome with Magnetic Resonance Correlation Pamela S. Chavis, M. D., Ali Al- Hazmi, M. D., David Clunie, M. D., and William F. Hoyt, M. D. Background: A young woman with a history of controlled hypertension noted a suddenly decreased peripheral temporal field in the left eye. This occurred after moderate peripartum hypertension. Method: A monocular peripheral temporal crescentic defect could be plotted on Goldmann visual fields despite a normal dilated peripheral retinal examination and normal disc appearance. Result: A dilated parieto- occipital sulcus could be seen on computed tomography, and magnetic resonance imaging showed changes consistent with atrophy and gliosis in the cu-neus, precuneus, and anterior calcarine cortex surrounding the parieto- occipital sulcus. Conclusion: By magnetic resonance imaging, this can be seen to comprise less than 10% of the visual cortex, as suggested by the Horton and Hoyt revised Holmes map. The temporal crescent syndrome is a rare monocular retrochiasmatic visual field defect that can be correlated to a lesion along the parietooccipital sulcus. Key Words: Temporal crescent syndrome- Peripheral vision- Magnetic resonance. Temporal crescent syndrome, or half- moon syndrome, is a rare disorder manifested by sudden, unilateral loss of vision in the temporal peripheral field. The anatomy of the visual cortex and visual fields allow for the monocular representation of the peripheral 30° of temporal field, and this is the only clinical example of a monocular retrochiasmatic visual pathway lesion ( 1). A complete temporal crescent defect is rare and has not been observed in several retrospective reviews of homonymous hemianopia ( 2- 6). Even partial temporal crescent defects only lately have been correlated to computed tomography ( CT) or magnetic resonance ( MR) imaging of the Manuscript received December 12, 1996. From the Departments of Ophthalmology ( P. S. C., A. A.- H.) and Radiology ( D. C.), King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, and the Department of Neurological Surgery, Neuro- Ophthalmology Unit ( W. F. H.), University of California, San Francisco, California, U. S. A. Address correspondence and reprint requests to Dr. Pamela S. Chavis, King Khaled Eye Specialist Hospital, PO Box 7191, Riyadh 11462, Kingdom of Saudi Arabia. Presented at the North American Neuro- Ophthalmologic Society Meeting, Snowbird, Utah, February 1996 as a poster. striate cortex ( 7). Sparing of this area has been reported ( 5,8- 11). We present a typical manifestation of this syndrome in a patient who presented shortly after moderate peripartum hypertension with a complaint of peripheral loss of vision. CASE REPORT History A 23- year- old Saudi woman noted sudden loss of vision in the left temporal periphery accompanied by diffuse headache. This had occurred 40 days before presentation and was immediately subsequent to a normal delivery that was complicated by moderate peripartum hypertension with blood pressure 180/ 110 mm Hg; concomitant severe diffuse headache and pedal edema were present, but seizures, alterations of consciousness, visual hallucinations weakness, or numbness were denied. She had a prior history of mild hypertension, which had been controlled. She is para 3, gravida 3. Examination Neurologic examinations gave normal findings, including normal carotid pulses without bruit or thrill. There were no cardiac murmurs. Visual acuity was 20/ 20 OU; intraocular pressure was 14 mm Hg OU. Biomicroscopic examination revealed normal anterior segment OU. Pupil light response was 3.5/ 3.5 + 2 without a relative afferent pupillary defect. Results of testing with the American Optical Hardy Rand Rittler color plates were normal OU. There was neither nystagmus nor spasticity of conjugate gaze. Ocular pursuit and saccade were normal; optokinetic nystagmus was normal and symmetric. Fundus examination gave normal results in both eyes including the peripheral retina. Goldmann visual field OS showed a temporal crescentic peripheral field defect, but OD was normal ( Fig. 1). Laboratory Data Complete blood count, erythrocyte sedimentation rate, antinuclear antibody, and serum protein electrophoresis all were normal. Pattern visual evoked responses were normal. The CT scan on presentation, 40 days after the onset of symptoms, revealed a band of focal low density 151 152 P. S. CHAVIS ETAL. 180 5a 120 90 60 2S 0 180 2a 240 270 300 1 5 0 / / 90 7 nNl ) 210\ \ •> 01 1 m \ L& \ \ ± \ \ i ^ 0[ 2 "-\- J 0 A o\ \ r T w ^ v p4eV^ 1° \ A30 J- liS TF 0 \ 90 / / 3 3 0 240 270 300 Left Right FIG. 1. Goldmann visual field OD normal and OS with a peripheral temporal crescent loss. FIG. 2. Magnetic resonance imaging of three parasagittal images: T1 ( 630/ 15)- weighted ( A and B) and T2 ( 2400/ 80)- weighted ( C) spin- echo images of the occipital lobe demonstrate atrophy and gliosis of the precuneus and cuneus adjacent to the parieto- occipital sulcus ( arrows) and preservation of the primary visual cortex and calcarine sulcus ( arrowheads). Notice the flow void in the branch of the parieto- occipital artery. J Neuro- Ophlhalmol, Vol. 17, No. 3, 1997 TEMPORAL CRESCENT SYNDROME 153 in the medial right occipital lobe with dilation of adjacent sulci. The MR imaging scans 6 and 18 months later demonstrated focal enlargement of the entire length of the parieto- occipital sulcus and a normal appearance of the calcarine sulcus on both short TR ( Tl- weighted) and long TR long TE ( T2- weighted) spin- echo sequences. The dilated parieto- occipital sulcus was surrounded by a thin band of increased signal on long TR short TE ( proton density- weighted) spin- echo sequences ( Fig. 2). Cortical T2- weighted spin- echo sequences also showed a lesion at the terminal branch of the posterior temporal artery ( left side) and more involvement at the terminal branches of the parieto- occipital and calcarine arteries from the posterior cerebral artery ( right side) ( Fig. 3). DISCUSSION Although this visual field defect involved only the peripheral 30° crescent in one eye, it was acute in onset and caused the patient to seek medical advice. Gradual defects, however, may progress more slowly and be ignored. The temporal crescent extends beyond 90°, but Goldmann perimetry does not allow for mapping of the most temporal portion. This perimetric region does not " localize to the parieto- occipital sulcus" but rather adjacent to it at the junction with the calcarine sulcus. The beginning of an understanding of the cortical representation of the visual field was achieved by correlating visual field defects to wartime injuries ( 12,13). The periphery of the visual field was mapped to the anterior visual cortex at the junction of the parieto- occipital and calcarine fissures, but the visual fields were plotted with a hand- held perimeter ( which is more reliable for central fields than peripheral) ( 9). The accuracy of the Holmes map was confirmed by CT for central fields, but traditional concepts of the temporal crescent syndrome may need revision ( 5,9,14). Topographic mapping of the primary visual cortex by MR imaging suggests that only 10% constitutes the far periphery of visual field and the most anterior lesion ( 15). Hence, a revised map of the representation of visual field in human striate cortex was produced in which the area serving central vision was expanded and the area devoted to peripheral vision was reduced ( 10) ( Fig. 4). Allowing for individual variability, Horton and Hoyt summarized the striate lesion into the following types: 1. An anterior lesion that lies adjacent to the parietooccipital fissure and affects the monocular temporal crescent of the contralateral visual field; this constitutes less than 10% of the striate cortex area ( supplied by the parieto- occipital artery) ( 16). 2. A posterior lesion that is located in the posterior 50% to 60% of the striate cortex and affects macular vision in central 10° of contralateral hemifield ( supplied totally by the calcarine artery in 50% of human brain) ( 16- 18). 3. An intermediate lesion that lies between the anterior and posterior confines and affects from 10° to 60°, which constitutes 30% of striate cortex ( 10). FIG. 3. Magnetic resonance imaging of lesion representing the terminal blood supply of the posterior temporal artery ( left) and, more notably, the terminal branches of the parieto- occipital and calcarine arteries from the posterior cerebral artery ( right); there also is a frontal white matter lacunar infarct ( right). ( Reproduced with permission from Stark DD, Bradley WG Jr. Magnetic resonance imaging, vol 1. St Louis: Mosby- Year Book, 1992: 599.) J Neiirii- Ophtluiliiitil. Vol. 17, No. J. 1997 154 P. S. CHAVIS ETAL. Left Visual Cortex Right Visual Field 90 Ijower Vertical Meridian - 2 7 0 - Mww Vertical t * e * » a I ' 1180 270 FIG. 4. Revised map of the representation of the visual field in the human striate cortex. ( Reproduced with permission from Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. Arch Ophthalmol 1991 ; 109: 816- 24.) A dilated parieto- occipital sulcus on routine imaging may be a clue to an associated anterior calcarine cortex lesion. The MR imaging in this patient demonstrates changes that are consistent with atrophy and gliosis of the cortex in the cuneus, precuneus, and anterior calcarine cortex surrounding the parieto- occipital sulcus and in the distribution of terminal branches of the parietal-occipital and calcarine branches of the posterior cerebral artery, as well as some posterior temporal artery involvement ( 19) ( Figs. 2 and 3). A linear flow void seen within the parieto- occipital sulcus may represent a patent terminal branch of the parieto- occipital artery. An additional focal lesion seen on the MR images was located in the deep white matter of the left frontal lobe, measuring 5 mm in diameter and demonstrating increased signal on long TR spin- echo sequences. At no time was hemorrhage, mass effect, or abnormal enhancement seen on either the CT or MR images. The extent of the lesions has remained unchanged over 18 months. Although this patient was not eclamptic, a hypertensive disorder was present; there was a vasculopathy with hypoxia and ischemia, and an additional lesion also was present in the deep white matter of the left frontal lobe. Visual disturbances from a vasculopathy such as eclampsia or hypotensive watershed lesions have been correlated to bilateral symmetric lesions in the posterior parietal and occipital areas, and other lesions have been noted in the frontal lobe and basal ganglion; such visual system lesions are less than 3 cm long, serpentine along the cortex, and do not produce a mass effect ( 20,2]). Certainly, MR imaging is not routinely done in preeclampsia. Several clinical concepts should be remembered. Monocular peripheral temporal visual field defects probably are caused more often by retinal lesions than by cortical lesions; thus, the nasal retinal periphery should be carefully examined ophthalmoscopically. Since these defects begin approximately 60° from fixation, the central field is normal and is insufficient to identify such a defect. REFERENCES 1. Walsh TX Temporal crescent or half- moon syndrome. Ann Ophthalmol 1974; 6: 501- 5. 2. Smith JL. Homonymous hemianopia: a review of one hundred cases. Am J Ophthalmol 1962; 53: 616- 23. 3. Trobe JD, Lorber ML, Schlezinger NS. Isolated homonymous hemianopia: a review of 104 cases. Arch Ophthalmol 1973; 89: 377- 81. 4. Fujino T, Kigazawa K, Yamada R. Homonymous hemianopia: a retrospective study of 140 cases. Neuroophthalmology 1986; 6: 17- 21. 5. Kattah JC, Dennis P, Kolsky MP, Schellinger D, Cohan SL. Computed tomography in patients with homonymous visual field defects: a clinico- radiologic correlation. Comput Tomogr 1981 ; 5: 301- 12. 6. McAuley DL, Ross Russell RW. Correlation of CAT scan and ./ Neiim- Opluluilnwl, Vol. 17, No. 3, 1997 TEMPORAL CRESCENT SYNDROME 155 visual field defects in vascular lesions of the posterior visual pathways. J Neurol Neurosurg Psychiatry 1979; 42: 298- 311. 7. Landau K, Wichmann W, Valavanis A. The missing temporal crescent. Am J Ophthalmol 1995; 119: 345- 9. 8. McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol 1994; 78: 185- 90. 9. Spector RH, Glaser JS, Noble JD, Vining DQ. Occipital lobe infarctions: perimetry and computed tomography. Neurology 1981; 31: 1098- 106. 10. Horton JC, Hoyt WF. The representation of the visual field in human striate cortex: a revision of the classic Holmes map. Arch Ophthalmol 1991; 109: 816- 24. 11. Miller NR. Walsh and Hoyt's clinical neuro- ophthalmology. 4th ed. Baltimore: Williams & Wilkins, 1982. 12. Holmes G, Lister WT. Disturbances of vision from cerebral lesions, with special reference to the cortical representation of the macula. Brain 1916; 39: 34- 73. 13. Spalding JMK. Wounds of the visual pathway. Part II. The striate cortex. J Neurol Neurosurg Psychiatry 1952; 15: 169- 83. 14. Fox PT, Miezin FM, Allman JM, Van Essen DC, Raichle ME. Retinotopic organization of human visual cortex mapped with positron- emission tomography. J Neurosci 1987; 7: 913- 22. 15. Stensaas SS, Eddington DK, Dobelle WH. The topography and variability of the primary visual cortex in man. J Neurosurg 1974; 40: 747- 55. 16. Kaul SN, Du Boulay GH, Kendall BE, Ross Russel RW. Relationship between visual field defect and arterial occlusion in the posterior cerebral circulation. J Neurol Neurosurg Psychiatry 1974; 37: 1022- 30. 17. Margolis NT, Newton TH, Hoyt WF. Cortical branches of the posterior cerebral artery: anatomic- radiologic correlation. Neuroradiology 1971; 2: 127- 35. 18. Smith CG, Richardson WFG. The course and distribution of the arteries supplying the visual ( striate) cortex. Am J Ophthalmol 1966; 61: 1391- 6. 19. Stark DD, Bradley WG Jr. Magnetic resonance imaging, vol 1. St Louis: Mosby- Year Book, 1992: 599. 20. Sanders TG, dayman DA, Sanchez- Ramos L, Vines FS, Russo L. Brain in eclampsia: MR imaging with clinical correlation. Radiology 1991; 180: 475- 8. 21. Wilson WB, Diiesbach JN, Shields RL. Watershed visual field loss: perimetry update. In: Kugler AH, Ghedini, eds. Proceedings of the VIII international Perimetric Society meeting. Amsterdam, Berkeley, Milano. J Neuro- Ophlhabnol, Vol. 17. No. J, 1997 |