| OCR Text |
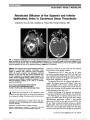
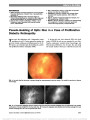
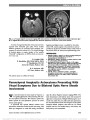
Show Literature Commentary Massey LA, Jäger HR, Paviour DC, O'Sullivan SS, Ling H, Williams DR, Kallis C, Holton J, Revesz T, Burn DJ, Yousry T, Lees AJ, Fox NC, Micallef C. The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856-1861. Objectives: Magnetic resonance imaging (MRI)-based measurements used to diagnose progressive supranuclear palsy (PSP) typically lack pathologic verification and are not easy to use routinely. We aimed to develop in histolog-ically proven disease a simple measure of the midbrain and pons on sagittal MRI to identify PSP. Methods: Measurements of the midbrain and pontine base on T1 midsagittal MRI were performed in confirmed PSP (n = 12), Parkinson disease (n = 2), and multiple system atrophy (MSA) (n = 7), and in controls (n = 8). Using receiver operating characteristic curve analysis, cutoff values were applied to a clinically diagnosed cohort of 62 subjects that included PSP (n = 21), Parkinson disease (n = 10), MSA (n = 10), and controls (n = 21). Results: The mean midbrain measurement of 8.1 mm was reduced in PSP (P , 0.001) with reduction in the midbrain to pons ratio (PSP smaller than MSA; P , 0.001). In controls, the mean midbrain ratio was approximately two-thirds of the pontine base, in PSP it was ,52%, and in MSA the ratio was greater than two-thirds. A midbrain measurement of,9.35 mm and ratio of 0.52 had 100% spec-ificity for PSP. In the clinically defined group, 19 of 21 PSP cases (90.5%) had a midbrain measurement of ,9.35 mm. Conclusions: We have developed a simple and reliable measurement in pathologically confirmed disease based on the topography of atrophy in PSP with high sensitivity and specificity that may be a useful tool in the clinic. Neurodegenerative diseases with Parkinsonism have overlapping features and are sometimes difficult to diagnose with clinical certainty. Previous studies distinguishing pro-gressive supranuclear palsy (PSP) from multiple system atrophy (MSA) have provided useful magnetic resonance imaging (MRI) guidance, such as the "hummingbird" sign in PSP, not seen in MSA. However, there is little pathologic verification of the diagnoses in previous studies. The current study looked at the anterior-posterior diameter of the mid-brain and pons and a ratio of the 2 brainstem regions and correlated these findings to histopathology. The measures were highly reliable at distinguishing PSP from MSA and are more reassuring because of the pathologic confirmation. -Mark L. Moster, MD I recently saw a 56-year-old woman who was diagnosed with a cerebellar ataxia by the referring neurologist, but he noted that her smooth pursuit was not normal. She had been falling for a few years and her speech was mildly slurred. Her saccades were slowed, especially in the vertical plane, and she failed the 3-clap test. My impression was that she likely had very early PSP, but the neurologist was not too excited about that diagnosis. Since PSP is a clinical diagnosis, it can be challenging to convince both patients and providers of it in the early stages. After reading this article, I went back and measured her midsaggital brain MRI. Her midbrain was 8.8 mm, and her midbrain to pons ratio was 47%, further supporting my initial impression. The methodology is straightforward, and it took me less than 5 minutes to measure. This could represent a high-yield finding. -Michael S. Lee, MD Merchant KY, Su D, Park SC, Qayum S, Banik R, Liebmann JM, Ritch R. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013;Pii:S0161-6420(12) 01257-2. doi: 10.1016/j.ophtha.2012.12. 035. epub ahead of print. Objective: To assess the value of enhanced depth imaging optical coherence tomography (EDI OCT) in diagnosing and evaluating optic nerve head drusen (ONHD) compared with conventional diagnostic methods. Design: Prospective, comparative, cross-sectional study. Participants: Thirty-four patients with clinically visible or suspected ONHD in either eye based on dilated optic disc examination or optic disc stereophotography and without ocular comorbidity. Methods: Spectral-domain OCT of the optic nerve head in both conventional (non-EDI) and EDI modes, ultrasound B-scan, and standard automated perimetry were performed on both eyes of all participants. Main Outcome Measures: Detection and findings of ONHD between EDI OCT and conventional diagnostic methods. 302 Moster and Lee: J Neuro-Ophthalmol 2013; 33: 302-306 Literature Commentary Section Editors: Mark L. Moster, MD Michael S. Lee, MD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Results: Sixty-eight eyes were clinically classified into 3 groups: 32 eyes with definite ONHD, 25 eyes with sus-pected ONHD, and 11 normal-appearing fellow eyes. In the definite ONHD group, EDI OCT, non-EDI OCT, and ultrasound B-scan were positive for ONHD in all eyes, and visual field (VF) was abnormal in 24 eyes. In the sus-pected ONHD group, EDI OCT, non-EDI OCT, ultra-sound B-scan, and VF were positive in 17, 14, 7, and 3 eyes, respectively; 8 eyes had no evidence of ONHD in any of the tests. In normal-appearing fellow eyes, EDI OCT, non-EDI OCT, ultrasound B-scan, and VF were positive in 3, 1, 1, and 0 eyes, respectively; 4 eyes had no evidence of ONHD in any of the tests. Enhanced depth imaging OCT had a signif-icantly higher ONHD detection rate than ultrasound B-scan in all eyes (52/68 eyes vs 40/68 eyes; P , 0.001), in eyes with clinically suspected ONHD or normal-appearing fellow eyes (20/36 eyes vs 8/36 eyes; P , 0.001), and in eyes with clinically suspected ONHD (17/25 eyes vs 7/25 eyes; P = 0.002). Enhanced depth imaging OCT-detected ONHD appeared as signal-poor regions surrounded by short, hyper-reflective bands or isolated/clustered hyperreflective bands without a signal-poor core. In non-EDI OCT, posterior sur-faces of the ONHD and deep-seated hyper-reflective bands were invisible or less clear than in EDI OCT. Conclusions: Enhanced depth imaging OCT detects le-sions likely representing ONHD more often and better assesses their shape and structure than conventional tests. At a recent neuro-ophthalmologist meeting, I heard a speaker say that one should obtain a B-scan ultrasound for children suspected of papilledema. She stated that if it were normal, then the child should undergo neuroimaging and a lumbar puncture, suggesting that B-scan should easily identify optic nerve head drusen (ONHD). Enhanced depth imaging optical coherence tomography is a specialized algorithm on the Spectralis OCT (Heidel-berg Engineering, Germany), which improves imaging of deeper structures in the posterior segment. The authors compared the ability of non-EDI OCT, EDI-OCT, and B-scan ultrasound to identify ONHD among patients with definite ONHD, clinically suspicious ONHD, and normal appearing optic discs. Among the clinically suspicious and normal appearing optic discs with ONHD, EDI-OCT identified ONHD significantly better (20/20) than the non-EDI OCT (15/20) and B-scan ultrasound (8/20) among the groups with clinically suspicious and normal-appearing optic discs. None of the ONHD detected by ultrasound or non-EDI OCT were missed by the EDI-OCT. The authors describe the EDI-OCT findings of subtle ONHD as clusters of hyper-reflective bands posterior to Bruch membrane. Generally speaking, these subtle ONHD did not appear on ultrasonography. I would highly recommend taking a look at the figures to see exactly what they mean and how the ONHD appear using EDI-OCT. They are well worth studying, and I could envision future speakers stating that a child with suspected papilledema should undergo EDI-OCT (rather than B scan) to evaluate for ONHD. -Michael S. Lee, MD Advances in OCT technology continue to enhance the expertise of the clinician. The images in this article convincingly show ONHD and are reassuring that the patient does not have papilledema. The ability to demon-strate ONHD definitively at the time of clinical examina-tion relieves the stress and cost of a workup for papilledema. EDI-OCT in this preliminary study was better than our previous "gold standard" of ultrasonography for demon-strating ONHD. -Mark L. Moster, MD Armangue T, Titulaer MJ, Sabater L, Pardo-Moreno J, Gresa-Arribas N, Barbero-Bordallo N, Kelley GR, Kyung-Ha N, Takeda A, Nagao T, Takahashi Y, Lizcano A, Carr AS, Graus F, Dalmau J. A novel treatment-responsive encephalitis with frequent opsoclonus and teratoma. Ann Neurol. 2013. doi: 10.1002/ana.23917. epub ahead of print. Among 249 patients with teratoma-associated encephalitis, 211 had N-methyl-D-aspartate receptor antibodies and 38 were negative for these antibodies. While antibody-positive patients rarely developed prominent brainstem-cerebellar symptoms, 22 (58%) antibody-negative patients developed a brainstem-cerebellar syndrome, which in 45% occurred with opsoclonus. The median age of these patients was 28.5 years (12-41), 91% were women, and 74% had full recovery after immunotherapy and tumor resection. These findings uncover a novel phenotype of paraneoplastic opsoclonus, which until recently was likely considered "idiopathic" or "postinfectious". The triad, young age (teen-ager- young adult), systemicteratoma, and high response to treatment characterize this novel brainstem-cerebellar syndrome. The differential diagnosis of patients with opsoclonus most often includes parainfectious, paraneoplastic or idiopathic. This article moves some patients from the idiopathic category to the paraneoplastic group. The syndrome of brainstem-cerebellar dysfunction, often with opsoclonus, was seen in 38 patients with teratoma (mostly benign). This contrasts with the group of 211 with teratoma who had antibodies to the NMDA receptor and presented with psychosis or other behavioral abnor-malities and dyskinesias. The brainstem-cerebellar Moster and Lee: J Neuro-Ophthalmol 2013; 33: 302-306 303 Literature Commentary Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. presentation preceded the diagnosis of teratoma in 82%. Treatments included tumor resection, immunotherapy (pulsed IV methylpredisolone, IVIg). A teenager or young adult with a brainstem cerebellar syndrome, including opsoclonus, should now be evaluated for teratoma. These patients are older than most with neuroblastoma and younger than those with lung cancer. -Mark L. Moster, MD Let us also not forget that brainstem encephalitis with opsoclonus-myoclonus can also result from anti-Ri, Ma2, Hu, and amphiphysin associated with breast and ovarian cancer, small-cell lung cancer, and pediatric neuroblastoma. There is a nice review on evaluation of paraneoplastic disorders including teratomas from a European Federation of Neurological Societies task force (1). The article recom-mends transvaginal ultrasound as the first-line investigation for teratoma followed by computed tomography of the pelvis. -Michael S. Lee, MD 1. Titulaer MJ, Soffietti R, Dalmau J, et al. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18:19-e3. Morsch M, Reddel SW, Ghazanfari N, Toyka KV, Phillips WD. Pyridostigmine but not 3,4-diaminopyridine exacerbates ACh receptor loss and myasthenia induced in mice by muscle-specific kinase autoantibody. J Physiol. 2013;591(pt 10):2747-2762. doi: 10.1113/ jphysiol.2013.251827. epub February 25, 2013. In myasthenia gravis (MG), the neuromuscular junction is impaired by the antibody-mediated loss of postsynaptic acetylcholine receptors (AChRs). Muscle weakness can be improved on treatment with pyridostigmine, a cholinester-ase inhibitor, or with 3,4-diaminopyridine, which in-creases the release of ACh quanta. The clinical efficacy of pyridostigmine is in doubt for certain forms of myasthenia. Here we formally examined the effects of these compounds in the antibody-induced mouse model of anti-muscle-specific kinase (MuSK) MG. Mice received 14 daily injections of IgG from patients with anti-MuSK MG. This caused reductions in postsynaptic AChR densi-ties and in endplate potential amplitudes. Systemic deliv-ery of pyridostigmine at therapeutically relevant levels from days 7-14 exacerbated the anti-MuSK-induced structural alterations and functional impairment at motor endplates in the diaphragm muscle. No such effect of pyridostigmine was found in mice receiving control human IgG. Mice receiving smaller amounts of MuSK autoantibodies did not display overt weakness, but 9 days of pyridostigmine treatment precipitated generalized mus-cle weakness. In contrast, 1 week of treatment with 3,4-diaminopyridine enhanced neuromuscular transmis-sion in the diaphragm muscle. Both pyridostigmine and 3,4-diaminopyridine increase ACh in the synaptic cleft, yet only pyridostigmine potentiated the anti-MuSK-induced decline in endplate ACh receptor density. These results thus suggest that ongoing pyridostigmine treatment potentiates anti-MuSK-induced AChR loss by prolonging the activity of ACh in the synaptic cleft. In the healthy neuromuscular junction, MuSK may help promote growth of postsynaptic AchR, while acetylcholine may contribute to AchR pruning. Studies have suggested that patients with anti-MuSK MG may experience wors-ening with pyridostigmine use. In a series of elegant experiments, the authors showed that treatment with pyridostigmine worsened the findings in mice receiving anti-MuSK IgG injections: (1) mice became weaker clinically, (2) the endplate potential (EPP) amplitude of the diaphragm declined, and (3) AchR density declined compared to untreated mice receiving injections of anti- MuSK IgG. Normally, 3,4-DAP acts by increasing the number of Ach quanta released from the presynaptic terminal com-pared to pyridostigmine, which increases the duration of Ach within the cleft. Treatment with 3,4-DAP had no effect on clinical weakness or AchR density, but did improve EPP amplitude. This suggests that the synergistic effect of anti- MuSK IgG and pyridostigmine on AchR loss occurs from prolonged exposure of Ach rather than quantity of Ach. Clinically, sometimes I treat patients suspicious for MG with pyridostigmine before the laboratory testing results come back. This article will make me consider avoiding pyridostigmine among patients with bulbar symptoms who may be suspicious for anti-MuSK MG. I can understand that pyridostigmine can be ineffective in MuSK-positive patients, but who would have thought it could be detrimental? -Michael S. Lee, MD Michael, I would be very hesitant to make any clinical practice changes based on this article. First, the authors did not study mice treated with acetylcholine receptor anti-bodies (AchRAb), so one may not conclude that there are different effects of pyridostigmine in MuskAb MG patients than in AchRAb MG patients. Second, the dosages used in this study are well more than commonly used therapeutic doses in humans or in animal studies. Most of my patients are on 180-360 mg/d of pyridostig-mine, up to approximately 5 mg/kg/d, well below the 16 mg/kg/d in this study. It is well known that even in Ach- Rab MG, clinical AchRAb patients, worsening occurs with 304 Moster and Lee: J Neuro-Ophthalmol 2013; 33: 302-306 Literature Commentary Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. pyridostigmine overdosage, so we must use caution with this medication. -Mark L. Moster, MD I would agree with you, however it does give one pause that the clinical worsening could result from AchR loss caused by the pyridostigmine use. I think further study is necessary, but to me, it is an unexpected finding. -Michael S. Lee, MD Chaudhuri Z, Demer JL. Sagging eye syndrome: connective tissue involution as a cause of horizontal and vertical strabismus in older patients. JAMA Ophthalmol. 2013:1-7. doi: 10.1001/ jamaophthalmol.2013.783. epub ahead of print. Importance: Recognition of sagging eye syndrome (SES) as the cause of chronic or acute acquired diplopia may avert neurologic evaluation and imaging in most cases. Objectives: To determine whether SES results from inferior shift of lateral rectus (LR) extraocular muscle (EOM) pulleys and to investigate anatomic correlates of strabismus in SES. Design and Setting: We used magnetic resonance imaging to evaluate rectus EOMs, pulleys, and the LR-superior rec-tus (SR) band ligament at an eye institute. Participants: Patients with acquired diplopia suspected of having SES. We studied 56 orbits of 11 men and 17 women (mean [standard deviation] age, 69.4 [11.9] years) clinically diagnosed with SES. Data were obtained from 25 orbits of 14 control participants age-matched to SES and from 52 orbits of 28 younger controls (23 [4.6] years). Main Outcome Measures: Rectus pulley locations com-pared with age-matched norms and lengths of the LR-SR band ligament and rectus EOMs. Data were correlated with facial features, binocular alignment, and fundus torsion. Results: Patients with SES commonly exhibited blepharop-tosis and superior sulcus defect. Significant inferolateral LR pulley displacement was confirmed in SES, but the spec-trum of abnormalities was extended to peripheral displace-ment of all other rectus pulleys and lateral displacement of the inferior rectus pulley, with elongation of rectus EOMs (P , 0.001). Symmetrical LR sag was associated with diver-gence paralysis esotropia and asymmetrical LR sag greater than 1 mm with cyclovertical strabismus. The LR-SR band was ruptured in 91% of patients with SES. Conclusions and Relevance: Widespread rectus pulley dis-placement and EOM elongation, associated with LR-SR band rupture, causes acquired vertical and horizontal stra-bismus. Small-angle esotropia or hypertropia may result from common involutional changes in EOMs and orbital connective tissues that may be suspected from features evi-dent on external examination. This is an important article. It further expands the narrative that Joseph Demer, MD, has been developing regarding orbital causes of diplopia not because of neuro-logic illness and that occur with increasing frequency with aging. Currently many neuro-ophthalmologists perform MRIs on patients presenting with divergence insufficiency and most often do not find abnormalities that alter the treatment. If additional studies support the concept that benign structural changes in the orbit may cause diplopia, we may reach the point where, in many instances, imaging of the brain is no longer necessary. -Mark L. Moster, MD Interestingly, the authors did not actually define what constituted a diagnosis of SES. In the introduction and the discussion, I get the impression that it is defined by a "sag" or inferior displacement of the lateral rectus pulley, which leads to the clinical fidings and also requires MRI to define. Clinically, these patients may have ptosis, deep superior sulcus, or high eyelid creases. The horizontal ductions and saccadic speeds should be normal, but there may be a diver-gence insufficiency pattern. Others may exhibit vertical stra-bismus with significant unilateral or bilateral elevator palsy. To me, I would not feel comfortable diagnosing SES and avoiding neuroimaging especially in a patient who has ptosis and an elevator palsy on the same side as shown in one of their figures. Instead, I see the greatest value in their MRI findings. If I order neuroimaging in such a patient, I would look for the enlongation of the EOM and centrifugal displacement of the rectus pulleys. I do not know if the MRI would be good enough to see rupture of the LR-SR band, but I would look for it. The MRI findings could help confirm the diagnosis of SES as the probable causes of diplopia. -Michael S. Lee, MD Pushker N, Tejwani LK, Bajaj MS, Khurana S, Velpandian T, Chandra M. Role of oral corticosteroids in orbital cellulitis. Am J. Ophthalmol. 2013. pii: S0002-9394(13) 00104-9. doi:10.1016/j.ajo2013. epub ahead of print. Purpose: To evaluate the role of oral corticosteroids as an anti-inflammatory adjunct in the treatment of orbital cellulitis. Design: Prospective, comparative, single-masked, interven-tional clinical study. Methods: Setting: tertiary eye care center (All India Institute of Medical Sciences). Study population: patients with acute Moster and Lee: J Neuro-Ophthalmol 2013; 33: 302-306 305 Literature Commentary Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. onset (within 14 days) of orbital cellulitis with or without abscess. Intervention: patients were randomized into 2 groups in the ratio of 1:2. Both groups received initial intravenous antibiotics. In Group 2, oral steroids were added after an initial response to intravenous antibiotics. Main outcome measures: resolution of signs and symptoms, duration of intra-venous antibiotics, length of hospital stay, and sequelae of disease (ptosis, proptosis, and movement restriction) were evaluated and compared between the 2 groups. Results: A total of 21 patients (age range, 11-59 years) with orbital cellulitis were studied. There were 7 patients in Group 1, who received standard intravenous antibiotics, and 14 in Group 2, who received adjuvant steroids. Patients in Group 2 showed an earlier resolution of inflammation in terms of periorbital edema (P = 0.002 at day 7), conjunctival chemosis (P , 0.001 at day 10), and pain (P = 0.012 at day 7). They also attained vision of 0.02 on logarithm of the minimum angle of resolution earlier than Group 1 patients. Decrease in proptosis and improvement in extraoc-ular movements were also significantly better with the use of steroids (P = 0.027 at day 10, P = 0.003 at day 14, respec-tively). While a significant number of patients in Group 1 had mild residual ptosis, proptosis, and movement restriction at 12 weeks, none of the patients treated with steroids had any residual changes (P = 0.023, P = 0.001, and P = 0.001, respectively). The durations of intravenous antibiotics and hospital stay were significantly less in Group 2. Conclusions: Use of oral steroids as an adjunct to intrave-nous antibiotic therapy for orbital cellulitis may hasten res-olution of inflammation with a low risk of exacerbating infection. In this study, if patients responded to intravenous anti-biotics within the first 3 days, oral prednisone was begun at 1.5 mg/kg/d for 3 days, followed by 1 mg/kg/d for 3 days, then tapered over 1-2 weeks. I was a bit surprised to hear that at 12-week follow up, persistent ptosis (3/7) and motility restriction (6/7) were seen in the antibiotic alone group. (Keep in mind that motility restriction was defined as excur-sion of the eye measured in millimeters). The 14 patients receiving adjuvant corticosteroids did not have any residual ptosis or motility restriction. The authors did not elaborate on how the examiners were masked nor did they indicate whether diplopia accompanied the motility restriction, so there may have been bias. I have never really thought about adding corticosteroids to the treatment of orbital cellulitis, but certainly our ENT colleagues do it a lot when they treat bacterial sinusitis. The thought behind reducing inflammation as we treat infection makes sense, and I think it deserves further exploration. -Michael S. Lee, MD This small study showed a more rapid recovery and mildly better outcome in orbital cellulitis patients who received corticosteroids after antibiotics were on board. Whether the benefit is related to anti-inflammatory effect or to a decrease in swelling in a tight "compartment" is not clear. Importantly, they report "no increase in steroid-related adverse events" although no details are provided. These results are consistent with findings in other bacterial infections, such as sepsis and meningitis, where corticoste-roids are sometimes used. -Mark L. Moster, MD 306 Moster and Lee: J Neuro-Ophthalmol 2013; 33: 302-306 Literature Commentary Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |