| OCR Text |
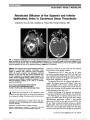
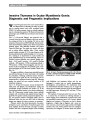
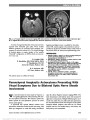
Show Perineural Optic Nerve Enhancement on Magnetic Resonance Imaging in Giant Cell Arteritis Katy C. Liu, PhD, David A. Chesnutt, MD Abstract: Giant cell arteritis (GCA) may cause visually devastating optic neuropathy. In atypical cases, diagnosis of optic neuropathy can be delayed. We present 2 such atypical cases and demonstrate that contrast-enhanced orbital magnetic resonance imaging may be a valuable tool in patient evaluation and aid in the diagnosis of GCA. Journal of Neuro-Ophthalmology 2013;33:279-281 doi: 10.1097/WNO.0b013e3182915b77 © 2013 by North American Neuro-Ophthalmology Society At times, patients with giant cell arteritis (GCA) may present without classic signs and symptoms. For exam-ple, in arteritic posterior ischemic optic neuropathy (PION), the fundus, including the optic disc, appears normal (1). This may lead to delay in diagnosis and treatment, often with devastating consequences. We evaluated 2 patients who illustrate this clinical conundrum and demonstrate the utility of dedicated fat-suppressed contrast-enhanced orbital magnetic resonance imaging (MRI) in the workup of unexplained vision loss in GCA. CASE REPORTS Case 1 An 83-year-old woman presented to her primary care physician with headache and blurred vision in her right eye. Visual acuity was hand motions, right eye, and 20/30, left eye. Funduscopy was normal bilaterally. Erythrocyte sedi-mentation rate (ESR) was 36 mm/hour with a platelet count of 494,000/mm3 (normal range: 150,000-400,000/mm3). C-reactive protein (CRP) was not obtained. Because GCA was suspected, the patient was given 250 mg of methylpred-nisolone intravenously every 6 hours beginning 90 minutes after ophthalmic examination. She was examined 14 hours later and found to have vision of hand motion, right eye, and light perception, left eye. The fundus examination remained unremarkable in both eyes. The patient received intravenous steroids for 3 days and then was switched to 60 mg of pred-nisone daily. The normal fundus appearance, with rapid progression of bilateral vision loss despite aggressive steroid treatment and normal ESR, prompted consideration of causes other than GCA. While brain MRI was normal, a dedicated orbital study demonstrated bilateral perineural optic nerve enhancement (Fig. 1). Additional workup for autoimmune, inflammatory, and neoplastic etiologies including cerebro-spinal fluid analysis was nondiagnostic. Temporal artery biopsy showed focal infiltration of the media with lympho-cytes and multinucleated giant cells, consistent with GCA. Four days later, visual acuity was no light perception in the right eye and light perception in the left eye. Over the following 2 weeks, visual acuity deteriorated to no light perception in both eyes, and remained unchanged over sev-eral months as her prednisone was gradually tapered. Case 2 A 68-year-old woman was evaluated by her local eye care provider with a 1-day history of blurred vision in her left eye. Visual acuity was 20/20, right eye, and 20/25, left eye. There was a left relative afferent pupillary defect and automated perimetry was normal in the right eye and showed superior and inferior arcuate defects in the left eye. The right fundus was normal, whereas the left fundus reportedly showed pallid optic disc swelling with disc elevation with peripapillary hemorrhages. Laboratory testing included ESR of 10 mm/hour, CRP of 1.1 ng/dL (normal range: 0.0-4.9 mg/dL) and platelet count of 202,000/mm3 (normal range: 150,000-400,000/mm3). Department of Ophthalmology (KCL, DAC), University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, North Carolina. Supported by Research to Prevent Blindness, National Institutes of Health (NIH) Grant 1F30DK089695-01 and NIH Grant T32 GM008719. The authors report no conflicts of interest. Address correspondence to David A. Chesnutt, MD, Department of Ophthalmology, University of North Carolina at Chapel Hill School of Medicine, CB# 7040, 5133 Bioinformatics Building, Chapel Hill, NC 27599-7040; E-mail: david_chesnutt@med.unc.edu Liu and Chesnutt: J Neuro-Ophthalmol 2013; 33: 279-281 279 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Acuity in the left eye declined to hand motions. Believed to have nonarteritic anterior ischemic optic neuropathy, the patient was referred nonurgently for neuro-ophthalmic evaluation. When seen in consultation 2 weeks later, visual acuity was 20/20, right eye, and no light perception, left eye. The right fundus was unremarkable, whereas the left disc was pale. The patient was treated with 60 mg of prednisone daily, and temporal artery biopsy was scheduled. Brain MRI with contrast was normal, but an orbital study revealed perineural enhancement of the left optic nerve (Fig. 2). Based on imaging studies, the patient was admitted to the hospital for further workup and given 1 g of methylpred-nisolone intravenously daily for 5 days and then placed on 60 mg of prednisone per day. Temporal artery biopsy showed lymphohistiocytic infiltrate and disruption of the internal elastica consistent with GCA. During 1 year of follow-up, the patient's vision has remained stable and her prednisone dosage has been gradually reduced. DISCUSSION Enhancement of the optic nerve on MRI may involve the nerve itself or the surrounding meninges. Optic nerve enhancement has been reported in a variety of disorders including optic neuritis (2,3), carcinomatous meningitis (3,4), idiopathic orbital inflammation (5), sarcoidosis (6), Wegener granulomatosis (7), and radiation-induced optic neuropathy (2). In contrast, perineural optic nerve enhancement is detected much less frequently. It has been described in optic perineuritis (nonspecific inflammation) (8), sarcoidosis (6), Wegener granulomatosis (7), syphilis (9), herpes zoster, and tuberculosis (10). In addition, there are 2 published reports of perineural enhancement in patients with GCA. Lee et al (11) described an 82-year-old woman with classic symptoms of GCA who had no light perception, left eye, with pallid optic disc edema. MRI revealed perineural enhancement of the left optic nerve, and temporal artery biopsy was consistent with GCA. Morgenstern et al (12) reported an 83-year-old man who presented with visual acuity of no light perception, right eye, and 20/50, left eye. He had bilateral pallid optic disc swelling, and MRI showed enhancement of both optic nerve sheaths and adjacent orbital fat. Positive temporal artery biopsies were obtained during his evaluation. In addi-tion, biopsy of the right optic nerve sheath showed inflam-mation of the perineural vasculature with multinucleated giant cells and disruption of the internal elastic lamina. FIG. 1. Case 1. Postcontrast axial (A) and coronal (B) T1 magnetic resonance imaging with fat suppression shows perineural enhancement of both optic nerves (arrows). FIG. 2. Case 2. Contrast-enhanced axial (A) and coronal (B) T1 magnetic resonance imaging with fat suppression demon-strate perineural enhancement of the left optic nerve (arrows). 280 Liu and Chesnutt: J Neuro-Ophthalmol 2013; 33: 279-281 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. It seems likely that arteritic PION played a role in the visual loss experienced by our patients. We propose that retrobulbar perineural enhancement signifying disruption of the blood-optic nerve barrier results from primary inflam-mation, secondary ischemia, or both. In our cases, the peri-neural pattern of the enhancement suggests that the more highly vascularized meningeal components of the nerve were preferentially affected. Inflammation in GCA injures endothelial cells that can increase vascular permeability of the paracellular and transcellular transport pathways regu-lated, in part, by endothelial tight junctions (13). Morgen-stern et al (12) have demonstrated active inflammation in the meshwork of pial vessels surrounding the optic nerve. The blood-optic nerve barrier can become highly permeable (14,15) and the optic nerve sheath expresses major histocom-patibility complex class II cells that participate in antigen pre-sentation in the inflammatory cascade (16). Retinal vessels preferentially seem to be spared in patients with GCA. It has been proposed that the retina is an "immune-privileged" organ, with retinal pigmented epithelial cells having several means of suppressing host immune responses (17-19). Thus, the breach of the blood-optic nerve barrier, but not the blood-retinal barrier, might explain why enhancement is restricted to the perineural space of the optic nerve. ACKNOWLEDGMENTS The authors acknowledge the following ophthalmology residents who helped to care for Case 1 during her inpatient stay: Robert van der Vaart, MD, Jonathan Zoghby, MD, Adam Dao, MD, and David Fleischman, MD. REFERENCES 1. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509-520. 2. Guy J, Mancuso A, Quisling RG, Beck R, Moster M. Gadolinium-DTPA-enhanced magnetic resonance imaging in optic neuropathies. Ophthalmology. 1990;97:592-599; discussion 599-600. 3. Tien RD, Hesselink JR, Szumowski J. MR fat suppression combined with Gd-DTPA enhancement in optic neuritis and perineuritis. J Comput Assist Tomogr. 1991;15:223-227. 4. Levy J, Marcus M, Shelef I, Lifshitz T. Acute bilateral blindness in meningeal carcinomatosis. Eye (Lond). 2004;18:206-207; discussion 207-208. 5. Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye (Lond). 2006;20:1196-1206. 6. Carmody RF, Mafee MF, Goodwin JA, Small K, Haery C. Orbital and optic pathway sarcoidosis: MR findings. AJNR Am J Neuroradiol. 1994;15:775-783. 7. Purvin V, Kawasaki A. Optic perineuritis secondary to Wegener's granulomatosis. Clin Exp Ophthalmol. 2009;37:712-717. 8. Purvin V, Kawasaki A, Jacobson DM. Optic perineuritis: clinical and radiographic features. Arch Ophthalmol. 2001;119:1299- 1306. 9. Frohman L, Wolansky L. Magnetic resonance imaging of syphilitic optic neuritis/perineuritis. J Neuroophthalmol. 1997;17:57-59. 10. Smith CH. Optic perineuritis. In: Miller NR, Newman NJ, eds. Walsh & Hoyt's Clinical Neuro-Ophthalmology, 6th edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2005:337-338. 11. Lee AG, Eggenberger ER, Kaufman DI, Manrique C. Optic nerve enhancement on magnetic resonance imaging in arteritic ischemic optic neuropathy. J Neuroophthalmol. 1999;19:235-237. 12. Morgenstern KE, Ellis BD, Schochet SS, Linberg JV. Bilateral optic nerve sheath enhancement from giant cell arteritis. J Rheumatol. 2003;30:625-627. 13. Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16: 209-221. 14. Tso MO, Shih CY, McLean IW. Is there a blood-brain barrier at the optic nerve head? Arch Ophthalmol. 1975;93:815-825. 15. Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia' is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201-1214. 16. Lassmann H, Rinner W, Hickey WF. Differential role of hematogenous macrophages, resident microglia and astrocytes in antigen presentation and tissue damage during autoimmune encephalomyelitis. Neuropathol Appl Neurobiol. 1994;20:195-196. 17. Wu GS, Rao NA. A novel retinal pigment epithelial protein suppresses neutrophil superoxide generation. I. Characterization of the suppressive factor. Exp Eye Res. 1996;63:713-725. 18. Wenkel H, Streilein JW. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Invest Ophthalmol Vis Sci. 2000;41:3467-3473. 19. Liversidge J, Forrester JV. Regulation of immune responses by the retinal pigment epithelium. In: Wolfensberger TJ, ed. The Retinal Pigment Epithelium: Function and Disease. New York, NY: Oxford University Press, 1998:551-527. Liu and Chesnutt: J Neuro-Ophthalmol 2013; 33: 279-281 281 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |