| OCR Text |
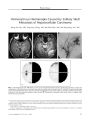
Show ORIGINAL CONTRIBUTION Orbital Metastasis of Hepatocellular Carcinoma Parima Hirunwiwatkul, MD, Suppapong Tirakunwichcha, MD, Piyawadee Meesuaypong, MD, and Shanop Shuangshoti, MD Abstract: We report a 74- year- old woman who presented with an orbital apex syndrome and pulsatile proptosis. CT showed a right orbital mass that destroyed the orbital sphenoid bone and extended intracranially Biopsy revealed metastatic hepatocellular carcinoma ( HCC), and subsequent investigations demonstrated a high level of serum- fetoprotein and a huge liver mass. The patient died shortly after the biopsy. Rarely reported in the English literature, metastatic HCC is common in Asia, perhaps because of a racial predisposition together with a relatively high prevalence of alcoholic cirrhosis, chronic hepatitis B and C, and exposure to anatoxins. (/ Neuro- Ophthalmol 2008; 28: 47- 50) etastatic tumor accounts for 3%- 7% of orbital neoplasms ( 1) and the common primary sources of tumor are carcinoma of the breast and lung. Hepatocellular carcinoma ( HCC) usually metastasizes to the lung, lymph nodes, adrenal gland, and bone. Orbital metastasis of HCC is rare, with only 14 biopsy- proven cases reported in the English literature ( 2- 15). Common presenting symptoms of orbital metastasis of HCC include pain, proptosis, and visual loss ( 2- 15). Most patients have been in an advanced stage of cancer and have typically died within 1 year of diagnosis. The purpose of this article is to report a case of orbital metastasis of HCC that caused an orbital apex syndrome and pulsatile proptosis. CASE REPORT A 74- year- old woman complained of headache and pain around the right eye for 2 months. She used antimigraine drugs that alleviated the pain. She denied proptosis and double vision. An ophthalmologist diagnosed Departments of Ophthalmology ( PH, ST, PW) and Pathology ( SS), Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Address correspondence to Parima Hirunwiwatkul, MD, Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, 1873 Rama IV Road, Patumwan Bangkok 10330, Thailand; E- mail: parima. h@ chula. ac. th glaucoma and cataract. Anti- glaucomatous drugs were dispensed. Her past medical history included diabetes mellitus, ischemic heart disease, and essential hypertension for 6 years. Her son and daughter had renal failure of unknown cause. She lived alone and had consumed alcohol heavily for 10 years. One week before her visit to us, she developed severe right- sided headache, rapidly progressive proptosis, deterioration of vision, and ptosis of the right eye. She reported a 7- kg weight loss within the past month. Ophthalmologic examination elsewhere disclosed intraocular pressures of 31 mm Hg in the right eye and 27 mmHg in the left eye, a right afferent pupillary defect, as well as proptosis ( Fig. 1) and reduced eye movements of the right eye in all directions. CT of the orbit showed a right orbital mass presumptively diagnosed as rhabdomyosarcoma ( Fig. 2). On our examination, visual acuity was hand movements in the right eye and finger counting in the left eye. Spontaneous pulsatile right proptosis and ptosis were observed on the right. She had orthophoria in primary position but the right eye had only 15% abduction and supraduction and 20% adduction and infraduction. The left FIG. 1. Two views of proptosis. J Neuro- Ophthalmol, Vol. 28, No. 1, 2008 47 J Neuro- Ophthalmol, Vol. 28, No. 1, 2008 Hirunwiwatkul et al FIG. 2. Precontrast ( A) and postcontrast ( B) orbit CT studies show a lobulated and uniformly enhancing mass in the lateral portion of the right orbit with bone destruction and extension to the anterior portion of middle cranial fossa. eye moved normally. The right pupil was dilated, with an afferent pupillary defect. Slit lamp examination revealed dense cataracts bilaterally, which precluded a view of the optic fundus. Decreased sensation was found on the right cornea and on the right side of face. There were no other neurologic deficits or lymphadenopathy detected. Orbital CT showed an 8 by 9 cm lobulated, hyper-dense, and uniformly- enhancing mass in the superolateral portion of the right orbit. The lesion extended into the anterior portion of the middle cranial fossa with bony destruction. Results of a complete blood count and standard blood chemistry analyses were within normal ranges except for elevated levels of serum glutamic oxaloacetic transaminase ( SGOT) ( 115 U/ L) and total bilirubin ( 1.57 mg/ dL). Biopsy of the orbital mass revealed polygonal- shaped tumor cells with vesicular nuclei and prominent nucleoli ( Fig. 3). The cells formed trabeculae rimmed by flat endothelium. Mitoses were occasionally encountered. Tumor cells stained positively with cytokeratin AE1/ AE3, HepPar- 1, and alpha- fetoprotein ( AFP). A pathologic diagnosis of metastatic HCC was rendered. Subsequent CT of the abdomen revealed a huge liver mass with retroperitoneal lymphadenopathy. The serum AFP level was markedly elevated ( 12,164 ng/ mL). These FIG. 3. Histopathology. A. Hematoxylin and eosin staining shows trabeculae of polygonal tumor cells rimmed by endothelium The tumor cells have vesicular nuclei and prominent nucleoli. B. HepPar- 1 immu-nostaining shows cytoplasmic granular positivity. 48 © 2008 Lippincott Williams & Wilkins Orbital Metastatic Hepatocellular Carcinoma J Neuro- Ophthalmol, Vol. 28, No. 1, 2008 findings further supported the diagnosis of HCC. Results of viral hepatitis profiles were negative. The patient received palliative treatment and died 2 months later. DISCUSSION Only 14 biopsy- proven cases of orbital metastasis of HCC have been reported in the English literature ( Table 1). No cases have been reported in large series from the United States and Europe ( 1,16,17), but in Japan, HCC ranks as the third most common cause of orbital metastatic cancers ( 18). This significant difference in orbital metastatic HCC is most likely due to the higher incidence of HCC in Asia. The incidence of HCC exceeds 30 in 100,000 in East Asia ( 19) and ranges between 1.2 and 8.8 in 100,000 in the United States ( 20) and 3 and 12 in 100,000 in Europe ( 21). Several risk factors for HCC development are frequently encountered in tropical Asian areas, particularly alcoholic cirrhosis ( 22), chronic hepatitis B and C ( 23- 25), and exposure to anatoxins ( 26), carcinogens produced by Aspergillus. Infection with hepatitis B virus is the major risk factor for HCC in Thailand, with an estimated relative risk of 15.2 ( 27). There have been many reports of an association between these risk factors and genetic abnormalities ( 28,29). Cai et al ( 30) have shown autosomal recessive inheritance of a major gene that influences the age of onset of HCC in eastern China. There have also been various studies dealing with the genetic predisposition to HCC among alcoholics. The alcohol dehydrogenase 1C* 1 allele has been found to be a genetic marker for alcohol-associated cancer in heavy drinkers ( 31). The presence of at least one alanine manganese superoxide dismutase ( Ala- MnSOD) allele increased the risk of development of cirrhosis in French alcoholics and increased the rate of TABLE 1. Fourteen reported cases Author Lubin et al ( 2) Zubler et al ( 3) Wakisaka et al ( 4) Phanthumchinda and Hemachuda ( 5) Tranfa et al ( 6) Schwab et al ( 7) Loo et al ( 8) Hosakawa et al ( 9) Font et al( 10) Scolyeret al ( 11) Kim et al ( 12) Gupta et al ( 13) Machado- Netto et al ( 14) Oidaetal( 15) Age/ Sex 69/ M 64/ M 58/ M 29/ W 85/ M 19/ M 71/ W 70/ M 79/ W 78/ M 56/ W 45/ M 57/ M 72/ M of biopsy- proven hepatocellular carcinoma with orbital metastasis Nationality American Japanese Thai Italian African Chinese Japanese Mexican Australian Korean Indian Brazilian Georgian Location of Metastasis Superoposterior orbit Lateral aspect of temporal fossa Frontal base extending to intraorbital space Superior orbital fissure Superotemporal orbit Superior orbit Anterior cranial fossa extending into orbit Lateral orbital wall Posterolateral orbital wall N/ A Lateral orbital wall Superotemporal orbit Temporal aspect of orbit Superolateral orbit Associated Risk Cirrhosis, alcoholism Alcoholism N/ A HBV infection N/ A N/ A HBV infection N/ A HCV infection N/ A HBV infection HBV infection HBV infection HCV infection Presenting Manifestations Progressive proptosis proptosis, ophthalmoplegia Diplopia on upward gaze Pain and ophthalmoplegia Pain and proptosis Proptosis with exposure keratitis Progressive visual loss Headache with visual disturbance Proptosis and visual loss Periorbital mass Inferior displacement of eye Proptosis Proptosis Diplopia, pain, proptosis M, male; F, female; N/ A, not available; HBY hepatitis B virus; HCy hepatitis C virus. 49 J Neuro- Ophthalmol, Vol. 28, No. 1, 2008 Hirunwiwatkul et al HCC development and death in cirrhotic patients ( 32). Alterations in genes involved in the RB1 and p53 pathways have been implicated in alcohol- related tumors ( 33). HCC can metastasize indirectly to the orbit via the skull base or directly to orbital tissue. The most common metastatic site within the orbit is the superotemporal region. Pain, proptosis, and visual loss are the typical presenting manifestations ( 5) ( Table 1). Bone destruction is common. Delayed diagnosis in our patient presumably allowed the mass to destroy the bone of the posterior orbit and create pulsatile proptosis, a manifestation not previously described. REFERENCES 1. Char DH, Miller T, Kroll S. Orbital metastases: diagnosis and course. Br J Ophthalmol 1997; 81: 386- 90. 2. Lubin JR, Grove AS Jr, Zakov ZN, et al. Hepatoma metastatic to the orbit. Am J Ophthalmol 1980; 89: 268- 73. 3. Zubler MA, Rivera R, Lane M. Hepatoma presenting as a retro-orbital metastasis. Cancer 1981; 48: 1883- 5. 4. Wakisaka S, Tashiro M, Nakano S, et al. Intracranial and orbital metastasis of hepatocellular carcinoma: report of two cases. Neurosurgery 1990; 26: 863- 6. 5. Phanthumchinda K, Hemachuda T. Superior orbital fissure syndrome as presenting symptom in hepatocellular carcinoma. J Med Assoc Thai 1992; 75: 62- 5. 6. Tranfa F, Cennamo G, Rosa N, et al. An unusual orbital lesion: hepatoma metastatic to the orbit. Ophthalmologica 1994; 208: 329- 32. 7. Schwab L, Doshi H, Shields JA, et al. Hepatocellular carcinoma metastatic to the orbit in an African patient. Ophthalmic Surg 1994; 25: 105- 6. 8. Loo KT, Tsui WM, Chung KH, et al. Hepatocellular carcinoma metastasizing to the brain and orbit: report of three cases. Pathology 1994; 26: 119- 22. 9. Hosokawa C, Kawabe J, Okamura T, et al. Usefulness of 99mTc- PMT SPECT and 18F- FDG PET in diagnosing orbital metastasis of hepatocellular carcinoma. Kaku Igaku 1994; 31: 1237- 42. 10. Font RL, Maturi RK, Small RG, et al. Hepatocellular carcinoma metastatic to the orbit. Arch Ophthalmol 1998; 116: 942- 5. 11. Scolyer RA, Painter DM, Harper CG, et al. Hepatocellular carcinoma metastasizing to the orbit diagnosed by fine needle aspiration cytology. Pathology 1999; 31: 350- 3. 12. Kim IT, Na SC, Jung BY. Hepatocellular carcinoma metastatic to the orbit. Korean J Ophthalmol 2000; 14: 97- 102. 13. Gupta R, Honavar SG, Vemuganti GK. Orbital metastasis from hepatocellular carcinoma. Surv Ophthalmol 2005; 50: 485- 9. 14. Machado- Netto MC, Lacerda EC, Heinke T, et al. Massive orbital metastasis of hepatocellular carcinoma. Clinics 2006; 61: 359- 62. 15. Oida Y, Ohtani Y, Dowaki S, et al. Hepatocellular carcinoma metastatic to the orbit: a case report. TokaiJExp Clin Med 2006; 31: 7- 10. 16. Shields JA, Shields CL, Brotman HK, et al. Cancer metastatic to the orbit: the 2000 Robert M. Curts Lecture. Ophthal Plast Reconstr Surg 2001; 17: 346- 54. 17. Ferry AP, Font RL. Carcinoma metastatic to the eye and orbit. I. A clinicopathologic study of 227 cases. Arch Ophthalmol 1974; 92: 276- 86. 18. Amemiya T, Hayashida H, Dake Y Metastatic orbital tumors in Japan: a review of the literature. Ophthalmic Epidemiol 2002; 9: 35^ 7. 19. Teo EK, Fock KM. Hepatocellular carcinoma: an Asian perspective. DigDis 2001; 19: 263- 8. 20. McGlynn KA, Tarone RE, El- Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic chol-angiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006; 15: 1198- 203. 21. Capocaccia R, Sant M, Berrino F, et al. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century.. Am J Gastroenterol 2007; 102: 1661- 70. 22. Voigt MD. Alcohol in hepatocellular cancer. Clin Liver Dis 2005 ; 9: 151- 69. 23. Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N EnglJMed 1993; 328: 1797- 801. 24. Lunn RM, Zhang YJ, Wang LY p53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res 1997; 57: 3471- 7. 25. Macias Rodriguez MA, Rendon Unceta P, Tejada Cabrera M. Risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Rev Esp Enferm Dig 2000; 92: 458- 69. 26. Mace K, Aguilar F, Wang JS. Aflatoxin Bl- induced DNA adduct formation and p53 mutations in CYP450- expressing human liver cell lines. Carcinogenesis 1997; 18: 1291- 7. 27. Srivatanakul P, Parkin DM, Khlat M et al. Liver cancer in Thailand. II. A case- control study of hepatocellular carcinoma. Int J Cancer 1991; 48: 329- 32. 28. Budhu AS, Zipser B, Forgues M. The molecular signature of metastases of human hepatocellular carcinoma. Oncology 2005; 69( Suppl l): 23- 7. 29. Laurent- Puig P, Zucman- Rossi J. Genetics of hepatocellular tumors. Oncogene 2006; 25: 3778- 86. 30. Cai RL, Meng W, Lu HY, et al. Segregation analysis of hepatocellular carcinoma in a moderately high- incidence area of east china. World J Gastroenterology 2003; 9: 2428- 32. 31. Homann N, Stickel F, Konig IR et al. Alcohol dehydrogenase 1C* 1 allele is a genetic marker for alcohol associated cancer in heavy drinkers. Int J Cancer 2006; 118: 1998- 2002. 32. Nahon P, Sutton A, Pessayre D et al. Genetic dimorphism in superoxide dismutase and susceptibility to alcoholic cirrhosis, hepatocellular carcinoma, and death. Clin Gastroenterol Hepatol 2005; 3: 292- 8. 33. Edamoto Y, Hara A, Biernat Wet al. Alteration of RBI, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer 2003; 106: 334^ 1. 50 © 2008 Lippincott Williams & Wilkins |