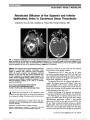
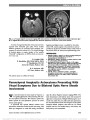
| OCR Text |
Show An Elderly Woman With Difficulty Reading and Abnormal Eye Movements Virginie Desestret, MD, PhD, Nathalie Streichenberger, MD, PhD, Muriel Panouillères, Denis Pélisson, PhD, B. Plus, MD, Charles Duyckaerts, MD, PhD, Dennis K. Burns, MD, Christian Scheiber, MD, PhD, Alain Vighetto, MD, Caroline Tilikete, MD, PhD Dr Tilikete A 73-year-old woman was evaluated in our neuro-ophthalmology clinic with a 1-year history of progressive difficulty reading. The patient's visual acuity, pupillary reactions to light and near stimulation, visual fields, and fundi were normal. Examination of her eye movements revealed a supranuclear vertical gaze abnormality, charac-terized by lack of upward saccades but intact downward saccades. The patient also had had difficulty initiating voluntary, especially leftward horizontal saccades on command, but reactive horizontal saccades were relatively well preserved. She was able to follow a pencil light moved by the examiner using small saccades (saccadic smooth pursuit) and her vestibulo-ocular reflex (VOR) was intact. She had apraxia of lid closure. The patient had no cognitive deficit, behavioral or social disturbance, aphasia, alexia, limb apraxia, postural ataxia, pyramidal signs or parkinsonism. Neuropsychological testing was hindered by reading difficulties but disclosed mild attentional and executive deficits, with verbal memory and language conserved (Table 1). Sac-cades were recorded (500 Hz; EyeLink II eye-tracker; SR Research, Mississauga, Canada) during a paradigm of reactive saccades toward 8 degrees right or left and a paradigm of voluntary scanning saccades during simultaneous presentation of three targets (28, 0, and +8 degrees). VOR during pendular chair stimulation (maximum velocity: 40 degrees per second, frequency: 0.25 Hz) and smooth pursuit (target amplitude: 30 degrees, frequency: 0.15 Hz) were recorded using 25-Hz infra-red video-oculography (VNG Ulmer; Synapsys, Marseille, France). The VOR was normal with preservation of the rightward and leftward quick phases (reflexive saccades) (Fig. 1A). Smooth pursuit showed saccadic following of the target (Fig. 1B). Reactive saccades to the right (Fig. 1C; Table 2) and left (Fig. 1D; Table 2) had normal latency and ampli-tude. Voluntary scanning saccades presented abnormal latency, specifically to the left (Fig. 1E, F; Table 2). Neuro-imaging included magnetic resonance imaging (MRI) of the brain, a SPECT scan, and a DaTscan. This last study is a SPECT scan for striatal dopamine transporter visualization. Dr Scheiber The brain MRI shows only frontal cortical atrophy without brainstem atrophy (Fig. 2A, B), and the standard SPECT study demonstrates bilateral frontal and left parietal hypo-perfusion (Fig. 2C, D). The DaTscan shows bilateral ni-grostriatal dopamine transporter loss (Fig. 2E). Dr Tilikete The patient was diagnosed with an acquired ocular motor apraxia and supranuclear vertical ophthalmoplegia, presumably from some type of degenerative process. There were no other signs of Parkinson disease or progressive supranuclear palsy (PSP). The differential diagnosis included fronto-temporal lobar degeneration (FTLD) and cortico-basal degeneration. Hospices Civils de Lyon, Service d'Anatomo-pathologie (VD, NS), Groupement Hospitalier Est, Bron, France; Hospices Civils de Lyon, Unité de Neuro-ophtalmologie and Service de Neurologie D (VD, AV, CT), Hôpital Neurologique, Bron, France; Université Claude Bernard Lyon I (NS, MP, DP, CS, AV, CT), Lyon, France; INSERM U1028 and CNRS UMR5292 (MP, DP, AV, CT), Lyon Neuroscience Research Center, IMPACT team, Lyon, France; Hospices Civils de Lyon, Service d'Explorations Fonctionnelles Neurologiques (BP), Hôpital de la Croix-Rousse, Lyon, France; Laboratoire de Neuro-pathologie R. Escourolle (VD, CD), La Salpêtrière Hospital, Paris, France; Department of Pathology (DKB), University of Texas Southwestern Medical Center, Dallas, Texas; Hospices Civils de Lyon, Service de Médecine Nucléaire (CS), Groupement Hospitalier Est, Bron France; and Centre de Neurosciences Cognitives (CS), UMR 5229-UCB Lyon 1 Prise de décision et Neuroéconomie Team, Lyon, France. Supported by the Hospices Civils de Lyon (HCL/P 2002.303). The authors report no conflicts of interest. Address correspondence to Caroline Tilikete, MD, PhD, Hospices Civils de Lyon, Unité de Neuro-ophtalmologie, Hopital Neuro-logique, 59 Boulevard Pinel, 69677 Bron Cedex, France; E-mail: caroline.tilikete@inserm.fr 296 Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 Clinical-Pathological Case Study Section Editor: Neil R. Miller, MD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. A vascular process was considered unlikely in view of the MRI findings. Subsequently, the patient developed bucco-facial apraxia, severe dysarthria, swallowing apraxia, and total vertical ophthalmoplegia, retaining dissociation between reactive and voluntary horizontal saccades. There was no postural imbalance, dementia, or signs of lower motor neuron disease (MND). Following her death 3 years after the initial presentation, a postmortem examination was per-formed according to the BrainNet Europe Consortium protocol (6). Autopsy consent was obtained in accordance with French and European regulations. Dr Burns The unfixed brain weighed 1,035 g and showed severe cortical atrophy affecting the fronto-temporal and parietal lobes. The basal nuclei and hippocampus showed less severe reduction in bulk. Pigmentation of the substantia nigra was normal. The cerebellum, cerebral peduncles, pontine base, medullary pyramids, and cervical spinal cord were unremarkable. An impressive histological feature visible in the sections of the cortex stained with hematoxylin and eosin is the presence of spongiform change, most conspicuous in the superficial cortical regions. Spongiform change is a feature of a number of different diseases, including neurodegenerative disorders, ischemia, and prion disease. Involvement of superficial cortical layers is characteristic of several neurodegenerative disorders, including Alzheimer disease and FTLD. Immuno-histochemical staining for various abnormal protein aggre-gates has become an essential part of the evaluation of FTLD and other neurodegenerative disorders. In the present case (Fig. 3), immunohistochemical evaluation demonstrates ubiquitin, TDP43, and the ubiquitin-binding protein p62 in the cytoplasm of both neurons and glial cells and within neuropil threads in the posterior frontal cortex, motor neu-rons in the brainstem (hypoglossal nucleus), and spinal cord, and in brainstem nuclei, including the rostral mesencephalic premotor oculomotor region. Ubiquitin reactivity is also present in neurons and neuropil threads in the pars compacta of the substantia nigra and in striatal fibers. No convincing TPD43 or ubiquitin reactivity is identified within the dentate gyrus of the hippocampus. The distribution of abnormal TDP43 and ubiquitin reactivity places this case in the cate-gory of TDP43/ubiquitin-related FTLD with MND, a vari-ant of FTLD that is often, but not invariably, associated with signs and symptoms of MND. Final Diagnosis FTLD of FTLD-TDP subtype 2, according to Sampathu FTLD classification (7). Dr Tilikete Our patient presented with a reading disorder that was related to a rare form of acquired, progressive ocular motor apraxia suggestive of frontal lobe dysfunction. Acquired ocular motor apraxia is clinically defined by loss of voluntary control of saccades and pursuit, with preservation of reflexive eye movements, including slow and quick phases (reflexive saccades) of vestibular nystagmus (i.e., VOR) (8). Conserva-tion of VOR slow phases and preservation of quick phases pointed to impaired cortical control of eye movements. The loss of initiation of saccades in this case was thought to reflect bilateral disruption of the descending neuronal ocular motor pathways from the frontal (FEF) and parietal (PEF) eye fields (9). The prominent alteration of leftward saccades suggested predominant right hemisphere involvement, and the dissoci-ated preservation of reactive saccades supported a prominent involvement of the (right) FEF pathway (10). This ocular motor disturbance corresponds to the mirror-model of the psychic paralysis of gaze or gaze apraxia observed in Balint syndrome, secondary to bilateral poste-rior parietal lobe lesions, in which voluntary saccades may be more easily initiated than reactive saccades (11). Clinical examination also revealed an upward vertical saccadic palsy, with preservation of downward saccades, vertical smooth pursuit, and VOR, consistent with a supranuclear vertical ophthalmoplegia. The absence of upward quick phases sug-gested tegmental brainstem involvement of premotor ocular TABLE 1. Neuropsychological evaluation Cognitive Function Testing Technique Testing Results Verbal memory Californian Verbal Learning Test (1) Delayed recall: 11/16 Total recall: 48/80 Recognition performance: 15/16 Language Protocol of Bachy- Langedock (2) 34/36 Visuospatial function, visual-motor integration Figures copies/praxia Score: 3/3 Attentional functions, executive functions Digits span (3) Digits backward: 3 Trail making test (4) A trial: 148 sec, ,10th percentile; B trial: not evaluable Stroop interference task (5) Coloras 47; 10' recall: 45 Verbal fluency Animals per minute: 17, P-words per minute: 6 Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 297 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. motor neurons (8). This progressive upward vertical oph-thalmoplegia associated with apraxia of lid closure met the criteria for possible PSP (12); however, the prominent ocu-lar motor apraxia in our patient differed from the more subtle impaired control of voluntary saccades (antisaccade errors) usually seen in patients with PSP (13). The clinical discrepancies between our patient's signs of FTLD and those of PSP were consistent with the lack of FIG. 1. Eye movement recordings. In all 6 graphs, horizontal eye position (full line) and target position (dashed line) in de-grees are presented relative to time in seconds. A. Vestibulo-ocular reflex during pendular chair stimulation is normal and leads to rightward and leftward (arrows) quick phases (reflexive saccades). B. Smooth pursuit shows saccadic tracking (arrows) of the target. Rightward (C) and leftward (D) reactive saccades appear normal in latency and amplitude. Rightward scanning voluntary saccades (E) have abnormal latency and amplitude, whereas leftward voluntary saccades (F) are absent and interrupted by a blink (arrow). TABLE 2. Gain and latency measurements (±SD) for rightward and leftward reactive and voluntary saccades Parameter Rightward Leftward Reactive saccades Gain 0.81 ± 0.05 (n = 21) 0.84 ± 0.05 (n = 20) Latency (ms) 205 ± 19 (n = 21) 254 ± 27 (n = 20) Voluntary saccades Gain 0.82 ± 0.32 (n = 26) 0.52 ± 0.26 (n = 15) Latency (ms) 564 ± 332 (n = 26) 1060 ± 521 (n = 15) 298 Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 2. Neuroimaging. Axial fluid-attenuated inversion recovery (A) and contrast-enhanced T1 sagittal (B) magnetic reso-nance imagings show frontal cortical atrophy without brainstem atrophy. No vascular or mass lesions are present. Sagittal (C) and coronal (D) brain SPECT scans reveal evidence of posterior frontal and left parietal hypoperfusion (arrows). Dat-SPECT imaging (E) discloses bilateral nigrostriatal dopamine transporter loss (arrows). FIG. 3. Neuropathological findings. A. The neocortices show gliosis and a variable degree of microvacuolation (hematoxylin and eosin). Immunohistochemical staining for ubiquitin, p62, and TDP-43 reveals numerous round glial (GCIs) and neuronal (NCIs) intracytoplasmic inclusions in the upper laminae of the frontal, temporal, and parietal cortices and in the striatum. B. TDP-43-positive NCIs. C. GCIs in striatal fiber fascicles which, on high magnification, contain coiled body-like TDP-43- positive oligodendroglial inclusions. TDP-43-positive NCIs and threads are present in brainstem nuclei, including the rostral mesencephalic premotor oculomotor region containing the interstitial nucleus of the medial longitudinal fascicle (D) and in anterior horn motor neurons (E). Skein-like and round NCIs are present in large neurons in the hypoglossal nucleus (F, G). Comma-shaped TDP-43-positive neurites were observed in the substantia nigra (H). Scale bars: 20 mm. Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 299 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. neuropathologic features of PSP. FTLD includes various neurodegenerative diseases characterized by selective degen-eration of the frontal and temporal lobes. Neuropathologi-cally, FTLD is associated with distinct pathological patterns, mainly abnormal accumulation of tau protein (FTLD-tau) or ubiquitin inclusions (FTLD-U subtype) (14). In FTLD-U, the majority of cases, as in our patient, show TDP-43 (TAR DNA-binding protein-43)-positive inclusions (FTLD-TDP) (15). Subtle and asymptomatic frontal ocular motor impair-ment (decreased horizontal saccade gain and increased anti-saccade errors) and PSP-like eye movement abnormalities have been demonstrated in FTLD, usually later in the clinical course (13,16,17). Primary symptomatic ocular motor impairment in FTLD is unusual. We found abnormal inclusions in the rostral portions of the medial longitudinal fasciculus, the premotor ocular motor relay for vertical saccades (18). These findings could explain the PSP-like supranuclear ophthalmoplegia. FTLD-TDP lesions were present in the FEFs as well as in modulators of the descending supranuclear pathways (striatum and sub-stantia nigra). These findings would explain the patient's ocular motor apraxia. Pathological analysis confirmed FTLD-MND, charac-terized by FTLD with TDP-43 inclusions, in a patient who did not develop signs of lower MND nor fronto-temporal dementia. According to Sampathu FTLD classification (7), this case was subtype 2, which often also exhibits TDP-43- positive inclusions in both upper and lower motor neurons (19). Motor neuron involvement is consistent with the high incidence of MND in these patients (20); however, clinical features of MND or dementia may be lacking in patients belonging to the neuropathological spectrum of FTLD-MND (21,22). The presence of specific inclusions in the hypoglossal nuclei but not in the ocular motor nuclei is also classically described in FTLD-MND patients (23). This pathological selectivity is partially explained by the fact that the TDP-43 proteinopathy may only affect nuclei that receive direct projections from cortical areas (24). The path-ophysiological vulnerability of some neuronal populations may determine the clinical phenotype. In conclusion, ocular motor apraxia previously has not been described as a predominant clinical syndrome associ-ated with FTLD. The findings in our patient broaden the clinical picture of FLTD with TDP-43 immunoreactive inclusions to include primary progressive ocular motor apraxia. ACKNOWLEDGEMENT The authors are grateful to C. Urquizar for his technical assistance. REFERENCES 1. Elwood RW. The California Verbal Learning Test: psychometric characteristics and clinical application. Neuropsychol Rev. 1995;5:173-201. 2. Bachy-Langedock N, Partz MP. Coordination of Two Reorganization Therapies in a Deep Dyslexic Patient With Oral Naming Disorder. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc, 1989. 3. Wechsler. Wechsler Adult Intelligence Scale. San Antonio, TX: The Psychological Corporation, 1997. 4. Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211-218. 5. Delis DC KE, Kramer JH. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation, 2001. 6. Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopoulou P, Kovács GG, Meyronet D, Parchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropath Exp Neurol. 2006;65:740-757. 7. Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VM. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Path. 2006;169:1343-1352. 8. Leigh RJ, Zee DS. The Neurology of Eye Movements, 4th edition, New York, NY: Oxford University Press, 2006. 9. Stuphorn V, Schall JD. Neuronal control and monitoring of initiation of movements. Muscle Nerve. 2002;26:326-339. 10. Sharpe JA, Johnston JL. Ocular motor paresis versus apraxia. Ann Neurol. 1989;25:209-210. 11. Pierrot-Deseilligny C, Gray F, Brunet P. Infarcts of both inferior parietal lobules with impairment of visually guided eye movements, peripheral visual inattention and optic ataxia. Brain. 1986;109:81-97. 12. Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1-9. 13. Garbutt S, Matlin A, Hellmuth J, Schenk AK, Johnson JK, Rosen H, Dean D, Kramer J, Neuhaus J, Miller BL, Lisberger SG, Boxer AL. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer's disease. Brain. 2008;131:1268-1281. 14. McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ; Work Group on Frontotemporal Dementia and Pick's Disease. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803-1809. 15. Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227-240. 16. Paviour DC, Lees AJ, Josephs KA, Ozawa T, Ganguly M, Strand C, Godbolt A, Howard RS, Revesz T, Holton JL. Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive neuronal changes: broadening the clinical picture to include progressive supranuclear palsy. Brain. 2004;127:2441-2451. 17. Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26:6354-6363. 300 Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 18. Rub U, Jen JC, Braak H, Deller T. Functional neuroanatomy of the human premotor oculomotor brainstem nuclei: insights from postmortem and advanced in vivo imaging studies. Exp Br Res. 2008;187:167-180. 19. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130-133. 20. Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathologica. 2006;112:539-549. 21. Zhang H, Tan CF, Mori F, Tanji K, Kakita A, Takahashi H, Wakabayashi K. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta Neuropathologica. 2008;115:115-122. 22. Nishihira Y, Tan CF, Onodera O, Toyoshima Y, Yamada M, Morita T, Nishizawa M, Kakita A, Takahashi H. Sporadic amyotrophic lateral sclerosis: two pathological patterns shown by analysis of distribution of TDP-43-immunoreactive neuronal and glial cytoplasmic inclusions. Acta Neuropathologica. 2008;116:169-182. 23. Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathologica. 2007;113:521-533. 24. Cook C, Zhang YJ, Xu YF, Dickson DW, Petrucelli L. TDP-43 in neurodegenerative disorders. Expert Opin Biol Ther. 2008;8:969-978. Erratum Are We There Yet? Is Neuro-Ophthalmology at the Cusp of a Paradigm Shift? Lessons From Leber Hereditary Optic Neuropathy: Erratum In the article that appears on page 189 of the June issue of the Journal of Neuro-Ophthalmology, Dr Simmons Lessell was misspelled as Simons Lessell in the first paragraph. REFERENCE 1. Sadun AA. Are We There Yet? Is Neuro-Ophthalmology at the Cusp of a Paradigm Shift? Lessons From Leber Hereditary Optic Neuropathy. J Neuroophthalmol. 2013; 189-197. Desestret et al: J Neuro-Ophthalmol 2013; 33: 296-301 301 Clinical-Pathological Case Study Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |