| OCR Text |
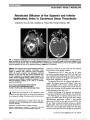
Show Posterior Reversible Encephalopathy Syndrome in a Leber Hereditary Optic Neuropathy Patient With Mitochondrial DNA 11778G.A Point Mutation Yuwei Da, MD, Xuxiang Zhang, MD, Fang Li, MD, Xiaoping Yang, MD, Xinqing Zhang, MD, Jianping Jia, MD Abstract: Leber hereditary optic neuropathy (LHON) is a maternally inherited mitochondrial disorder that primarily affects the optic nerve. We report a case of reduced visual acuity secondary to optic atrophy in a 13-year-old boy. Transient seizures developed subsequently. Serial magnetic resonance imaging of the brain showed posterior reversible encephalopathy syndrome. Ragged red fibers were not detected on skeletal muscle biopsy. A 11778G.A mitochon-drial DNA point mutation was identified in the lymphocytes isolated from peripheral blood. His younger brother was a car-rier with the same mutation. The presentation of this case is unusual documenting LHON in association with PRES. Journal of Neuro-Ophthalmology 2013;33:276-278 doi: 10.1097/WNO.0b013e31828f8d75 © 2013 by North American Neuro-Ophthalmology Society Leber hereditary optic neuropathy (LHON) is a mater-nally inherited mitochondrial disorder, characterized by sequential occurrence of acute or subacute bilateral visual loss and centrocecal scotomas. In most patients, there are no other neurologic manifestations, and neuroimaging of the brain is normal. However, additional neurologic features rarely have been described including the occurrence of LHON and multiple sclerosis (MS) (1-3). We describe a patient with symptoms compatible with LHON, con-firmed by genetic testing, who also had reversible bilateral occipital lesions associated with headache and epileptic seizures. CASE REPORT A 13-year-old boy developed blurred vision in his both eyes. He was the firstborn child of a healthy nonconsanguineous Chinese couple, and the family history was unremarkable. The patient initially was diagnosed with bilateral optic neuritis and underwent intravenous pulse methylpredniso-lone therapy without improvement in vision. Forty-seven days after the onset of symptoms, the patient complained of headache followed by 4 generalized tonic-clonic seizures. His blood pressure was normal, and there was no history of a seizure disorder. He was diagnosed with viral encephalitis and treated with acyclovir (50 mg every 8 hours for 2 weeks) and phenytoin (200 mg 3 times a day). Subsequently, he had no further seizures. On day 75 after the onset of symptoms, he was referred to our Neurology Department for further evaluation. Visual acuity was 20/400 bilaterally, visual fields showed central scotomas in each eye, and both optic discs were pale. Neurologic examination was otherwise normal. No evidence of abnormality was not found in complete blood count, urinalysis, biochemistries, including erythrocyte sedimentation rate and C reaction protein. Markers of autoimmune diseases were negative and included antinuclear antibody, antineutrophil cytoplasmic antibody, anticardioli-pin antibodies and rheumatoid factor. Examination of the cerebrospinal fluid (CSF) on 2 occasions was unremarkable. Serum and CSF viral antibody tests were negative for herpes simplex virus (HSV-1 and HSV-2), varicella zoster virus, Epstein-Barr virus, and measles virus. Viral polymerase chain reaction was not done on the CSF. Electroencephalography was normal. Magnetic resonance imaging (MRI) of the brain per-formed 42 days after the symptom onset, and prior to seizures, was normal. Five days following the onset of seizures, MRI revealed high signal intensity lesions Departments of Neurology (YD, FL, XZ, JJ); Ophthalmology (XZ); and Radiology (XY), Xuan Wu Hospital, Capital Medical University, Beijing, China. The authors report no conflicts of interest. Y. Da and X. Zhang have contributed equally. Address correspondence to Jianping Jia, Department of Neurology, Xuan Wu Hospital, Capital Medical University, Beijing 100053, China; E-mail: jiaxuanwu@126.com 276 Da et al: J Neuro-Ophthalmol 2013; 33: 276-278 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. involving the occipital lobes on both T2 and fluid-attenuated invasion recovery (FLAIR) images (Fig. 1A). Diffusion-weighted imaging showed no evidence of restricted diffusion. Sixteen days after the onset of seizures, there was partial resolution of the signal change in both occipital lobes (Fig. 1B), and by 34 days, MRI was normal in appearance (Fig. 1C). In evaluating our patient, we were suspicious of a mitochondrial disorder including LHON and mitochon-drial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS). Muscle biopsy (left brachial biceps) was performed, and no ragged red fibers were found. Screening mitochondrial DNA (mtDNA) for 3 primary LHON point mutations revealed a homoplasmic mtDNA G11778A mutation in lymphocytes. MELAS mtDNA point muta-tions (A3243G and A3271T) were absent. Large-scale rearrangements of mtDNA extracted from muscle were screened for Southern blotting of DNA and were not found. Sequencing the entire mtDNA coding region excluded rare mutations associated with MELAS, myoclonic epilepsy with ragged red fibers, or LHON/MELAS syn-drome. No other pathogenic mutations were found in all mtDNA transfer RNA, complex I, complex III, and complex V genes except for G11778A mutation. The diagnosis of LHON was established, and the patient was treated with coenzyme Q10. The presence of the G11778A mutation was confirmed in our patient's younger brother, who is clinically unaf-fected. The mother of the proband is asymptomatic and refused evaluation. DISCUSSION While the clinical presentation and results of genetic testing secured the diagnosis of LHON in our patient, the occurrence of seizures with LHON is rare (1,4). Serial brain MRI in our case revealed complete resolution of abnormal T2 and FLAIR signal changes in the occipital lobes, consistent with posterior reversible encephalopathy syndrome (PRES). PRES is characterized by seizures, altered mental state, headache, focal neurologic deficits, and visual disturbances (5-7). Cerebral edema most often is detected in the parieto-occipital region but may occur in the temporal lobe, tempo-ral- occipital junction, cerebellum, and, rarely, in the basal ganglia and brainstem. It commonly occurs at the border zones of cerebral arterial territories. Hypertension is the most frequent predisposing cause of PRES, but the syndrome has been reported in a wide variety of settings, including solid organ transplantation and medications used to prevent rejec-tion, renal disease, autoimmune disorders, severe infection, and mitochondrial disorders (8,9). The etiology of PRES in our patient is unclear. Headache and seizures also may occur in stroke-like episodes of MELAS. In addition, LHON/MELAS overlap phenotype associated with a complex I or complex V subunit gene mutation had been reported (10,11). How-ever, our patient did not have ragged red fibers on muscle biopsy, and the sequence analysis of entire mtDNA coding region revealed no other pathogenic mutations in all mtDNA transfer RNA, complex I, complex III, complex IV, and complex V genes. The most common mutations of LHON are missense mutation in mitochondrial encoded complex I subunits, which are 11778 G.A, 3460 G.A, and 14484 T.C. All of these mtDNA mutations have been reported to be associated with white matter disorders typically in a periventricular distribution resembling MS (4,12-15). Several pathogenic mechanisms have been proposed: 1) impairment of cellular energy genera-tion with ATP depletion; 2) this energy crisis causes lipid peroxidation and oxidative damage of protein, causing damage to the mitochondrial membrane; 3) altered mitochondrial per-meability leads to initiation of apoptotic cell death; and 4) vascular changes with the accumulation of abnormal mito-chondria in both endothelial and smooth muscle cells of the blood vessel walls (15,16). Magnetic resonance spec-troscopy studies of LHON with the G11778A mutation have demonstrated abnormal mitochondrial energy metab-olism in the occipital lobe (17). Grazina et al (12) FIG. 1. Serial axial fluid-attenuated inversion recovery magnetic resonance imaging obtained 5 (A), 16 (B), and 34 (C) days after the onset of seizures revealing an increased signal in both occipital lobes that gradually resolved. Da et al: J Neuro-Ophthalmol 2013; 33: 276-278 277 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. reported that in individuals harboring the 11778G.A mutation, possible association with other neurologic distur-bances may be explained by heteroplasmy, different tissue distribution of mutant mtDNA, and other genomic and envi-ronmental modifiers. It may be that a variety of these factors contributed to the unusual clinical course of our patient. ACKNOWLEDGMENTS The authors are grateful to the family members of the patients mentioned in this report for their collaboration. They thank Dr. Rabi Tawil (University of Rochester Medical Center) for critical reading of the manuscript and helpful suggestions. They are also grateful for the financial support from the Beijing Municipal Health Bureau on the "215" high-level health and technical personnel training project. REFERENCES 1. Newman NJ, Lott MT, Wallace DC. The clinical characteristics of pedigrees of Leber's hereditary optic neuropathy with the 11778 mutation. Am J Ophthalmol. 1991;111:750-762. 2. Horváth R, Abicht A, Shoubridge EA, Karcagi V, Rózsa C, Komoly S, Lochmüller H. Leber's hereditary optic neuropathy presenting as multiple sclerosis-like disease of the CNS. J Neurol. 2000;247:65-67. 3. Perez F, Anne O, Debruxelles S, Menegon P, Lambrecq V, Lacombe D, Martin-Negrier ML, Brochet B, Goizet C. Leber's optic neuropathy associated with disseminated white matter disease: a case report and review. Clin Neurol Neurosurg. 2009;111:83-86. 4. Nikoskelainen EK, Marttila RJ, Huoponen K, Juvonen V, Lamminen T, Sonninen P, Savontaus ML. Leber's "plus": neurological abnormalities in patients with Leber's hereditary optic neuropathy. J Neurol Neurosurg Psychiatry. 1995;59:160-164. 5. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500. 6. Powell ES, Goldman MJ. Posterior reversible encephalopathy syndrome (PRES) in a thirty-six-week gestation eclamptic. J Emerg Med. 2007;33:377-379. 7. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904-912. 8. Renard D, Bonnaure H, Labauge P. Teaching NeuroImages: diffuse posterior leukoencephalopathy in MELAS without stroke-like episodes. Neurology. 2010;75:e9. 9. Finsterer J, Stöllberger C, Ostermann E, Zuntner G, Huber J, Tscherney R. Recurrent posterior reversible encephalopathy syndrome in mitochondrial disorder. Blood Pres. 2009;18:126-129. 10. Blakely EL, de Silva R, King A, Schwarzer V, Harrower T, Dawidek G, Turnbull DM, Taylor RW. LHON/MELAS overlap syndrome associated with a mitochondrial MTND1 gene mutation. Eur J Hum Genet. 2005;13:623-627. 11. Pulkes T, Eunson L, Patterson V, Siddiqui A, Wood NW, Nelson IP, Morgan-Hughes JA, Hanna MG. The mitochondrial DNA G13513A transition in ND5 is associated with a LHON/ MELAS overlap syndrome and may be a frequent cause of MELAS. Ann Neurol. 1999;46:916-919. 12. Grazina MM, Diogo LM, Garcia PC, Silva ED, Garcia TD, Robalo CB, Oliveira CR. Atypical presentation of Leber's hereditary optic neuropathy associated to mtDNA 11778G.A point mutation-A case report. Eur J Paediatr Neurol. 2007;11:115-118. 13. Bhatti MT, Newman NJ. A multiple sclerosis-like illness in a man harboring the mtDNA 14484 mutation. J Neuroophthalmol. 1999;19:28-33. 14. Jansen PH, van der Knaap MS, de Coo IF. Leber's hereditary optic neuropathy with the 11778 mtDNA mutation and white matter disease resembling multiple sclerosis: clinical, MRI and MRS findings. J Neurol Sci. 1996;135:176-180. 15. Lev D, Yanoov-Sharav M, Watemberg N, Leshinsky-Silver E, Lerman-Sagie T. White matter abnormalities in Leber's hereditary optic neuropathy due to the 3460 mitochondrial DNA mutation. Eur J Paediatr Neurol. 2002;6:121-123. 16. Lerman-Sagie T, Leshinsky-Silver E, Watemberg N, Luckman Y, Lev D. White matter involvement in mitochondrial diseases. Mol Genet Metab. 2005;84:127-136. 17. Barbiroli B, Montagna P, Cortelli P, Iotti S, Lodi R, Barboni P, Monari L, Lugaresi E, Frassineti C, Zaniol P. Defective brain and muscle energy metabolism shown by in vivo 31P magnetic resonance spectroscopy in nonaffected carriers of 11778 mtDNA mutation. Neurology. 1995;45:1364-1369. 278 Da et al: J Neuro-Ophthalmol 2013; 33: 276-278 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |