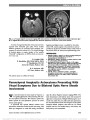
| OCR Text |
Show A Resting State Functional Magnetic Resonance Imaging Study of Patients With Benign Essential Blepharospasm Bo Zhou, Jinyu Wang, Yulan Huang, Yousong Yang, Qiyong Gong, Dong Zhou Background: Benign essential blepharospasm (BEB) is a neurologic disorder characterized by an adult-onset focal dystonia that causes involuntary blinking and eyelid spasms. The pathophysiology of BEB patients remains unclear. This study investigated intrinsic low-frequency fluctuation in BEB patients during resting state functional magnetic resonance imaging (fMRI). Methods: The study included 9 patients with BEB (mean age, 61.7 years; range, 52-66 years), in whom the average dura-tion of symptoms was 2.7 ± 1.8 years, and another 9 sub-jects from an age- and sex-matched control group. Resting state fMRI was performed in both the patients with BEB and the normal controls. Voxel-based analysis was used to char-acterize the alteration of amplitude of low-frequency fluctua-tion (ALFF) in both patients with BEB and the normal controls. Results: The whole brain analysis indicated that in compar-ison with the normal control group, there was a significantly increased ALFF in the left putamen, pallidum, insular lobe, and medial prefrontal cortex and a significantly decreased ALFF in the bilateral somatosensory regions, thalami, cerebellum, and medial and posterior cingulate cortex. Conclusion: The present study suggests that both an abnormal default mode network and corticostriatopallido-thalamic loop may play a role in the pathophysiology of BEB. Journal of Neuro-Ophthalmology 2013;33:235-240 doi: 10.1097/WNO.0b013e31828f69e5 © 2013 by North American Neuro-Ophthalmology Society Benign essential blepharospasm (BEB) is an adult-onset focal dystonia that causes involuntary blinking and eyelid spasms (1). Conventional neuroimaging methods, including magnetic resonance imaging (MRI), do not con-sistently show any structural abnormalities in patients with BEB. Studies using positron emission tomography (PET) (2,3) and magnetic resonance spectroscopy (4) have demon-strated abnormalities of basal ganglia and thalamic functioning in patients with BEB, and PET also showed hypoactivity in cortical areas controlling the eyelids and hyperactivity in the pons and cerebellum (5). Using functional MRI (fMRI), Schmidt et al (6) showed that the episodes of involuntary eyelid spasms in BEB patients correlate with an increased striatal activation, while Baker et al (7) demonstrated cortical and subcortical circuit abnormalities. Although these studies suggest that dysfunction in both the basal ganglia and cortical areas may be associated with BEB, the pathophysiology of this disorder remains poorly understood. Recently, there has been an increasing interest in the investigation of default mode of brain function. Through numerous neuroimaging studies (8-11), there is general consensus that default mode is a specific, anatomically defined brain system that is preferentially active when individuals are not focused on the external environment. This system is composed of the posterior cingulate cortex (PCC)/precuneus, ventral anterior cingulate cortex (vACC)/ medial prefrontal cortex (mPFC), inferior parietal lobe (IPL), and lateral and medial temporal structures. These regions constitute a default mode network (DMN) with close functional connectivity (12-16). Synchronous low-frequency fluctuation (LFF) in resting state fMRI has been extensively used to study spontaneous low-frequency neuronal activities. Some studies have sug-gested that the LFFs of blood oxygenation level-dependent (BOLD) fMRI signals are closely related to spontaneous neural activity (17-19). Although functional connectivity analysis can provide us with additional information of brain region networks, it does not reveal the BOLD signal change in regional spontaneous activity. Moreover, the detection of abnormal connectivity among brain regions does not tell us precisely the area of the brain with spontaneous abnormal Department of Neurology (BZ, JW, YH, YY), Sichuan Provincial People's Hospital, Chengdu, China; and Departments of Neurology (DZ) and Radiology (QG), West China Hospital, Sichuan University, Chengdu, China. Supported by the National Natural Science Foundation of China (grant nos. 30625024 and 30728017), programs from the State Education Ministry (grant no. SRFDP-20060610073), the National Basic Research Program (973 Program No: 2007CB512305/2), and the National High Technology Program of China (863 program no: 2008AA02Z408). The authors report no conflicts of interest. Address correspondence to Dong Zhou, Wai Nan Guo Xue Lane 37#, Chengdu, Sichuan 610041, China; E-mail: zhoudong6668@ yahoo.cn Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 235 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. activity. Biswal et al (20) developed an index called the amplitude of LFF (ALFF), for detecting the regional inten-sity of spontaneous fluctuations in BOLD signals, in which the square root of the power spectrum was integrated in a low-frequency range (,0.08 Hz). ALFF has already been used to study attention-deficit hyperactivity disorder (20,21), early stages of Alzheimer disease (22), and epilepsy (23). To our knowledge, there have been no studies of ALFF in BEB. We first analyzed intrinsic LFF in BEB patients during resting state by applying ALFF and then compared ALFF between BEB patients and normal controls. We hypothe-sized that BEB patients may have different ALFF in some brain areas in comparison with normal controls. MATERIALS AND METHODS Subjects This study analyzed 18 individuals including 9 patients with BEB and 9 normal volunteers from the Department of Neurology, West China Hospital, Sichuan University and the Sichuan Provincial People's Hospital in Chengdu. There were 2 men and 7 women with BEB, with a mean age of 61.7 years (range, 52-66 years). The average duration of symptoms was 2.7 ± 1.8 years. Two patients received botulinum toxin injections 6 months before the study, but symptoms recurred after 3 months. Patient met the follow-ing inclusion criteria: 1) BEB, 2) no previous neuroleptic medication, 3) no other neurologic or psychiatric disease, 4) normal MRI brain scan, and 5) right-handedness according to the Edinburgh Handedness Inventory (24). Normal con-trols included 4 men and 5 women in the same age range, all of whom were right-handed and not taking psychotropic drugs. All 18 participants gave their written informed con-sent according to the Declaration of Helsinki, and the study protocol was approved by the local ethics committee. Image Acquisition Experiments were performed on a 3.0-T GE-Signa MRI scanner (EXCITE; General Electric, Milwaukee,WI) in Huaxi MR Research Center (West China Hospital, Sichuan Univer-sity, Chengdu, China). Foam padding was used to minimize head motion for all subjects. Functional images were acquired using a single-shot, gradient-recalled echo planar imaging sequence (relaxation time [TR] = 2000 milliseconds, echo time [TE] = 30 milliseconds, and flip angle = 90°). Thirty transverse slices (field of view [FOV] = 24 cm, in-plane matrix = 64 · 64 mm, slice thickness = 5 mm, without gap, and voxel size = 3.75 · 3.75 · 5 mm), were acquired and aligned along the anterior commissure-posterior commissure line. For each sub-ject, a total of 205 volumes were acquired, and the first 5 volumes were discarded to ensure steady-state longitudinal magnetization. Subjects were instructed simply to rest with their eyes closed without falling asleep and thinking of nothing. Subsequently, for spatial normalization and localization, a set of high-resolution T1 anatomical images was acquired in axial orientation using a 3-dimensional spoiled gradient-recalled sequence (TR = 8.5 milliseconds, TE = 3.4 milliseconds, flip angle = 12°, matrix size = 512 · 512 · 156, and voxel size = 0.47 · 0.47 · 1 mm3) on each subject. Data Preprocessing Data preprocessing was carried out using SPM2 software (http://www.fil.ion.ucl.ac.uk/spm). The 200 volumes were first corrected for the temporal difference and head-motion correction. If translational or rotational parameters in a data set exceeded ±1.5 mm or ±1.5°, the data set was excluded from the analysis. There was no significant difference in the magnitude of motion correction parameters between the 2 participant groups (P . 0.05). The functional images were realigned with the corresponding T1 volume and warped into a standard stereotaxic space at a resolution of 3 · 3 · 3 mm3, using the Montreal Neurological Institute echo-planar imaging template in SPM2. Subsequently, they were spatially smoothed by convolution with an isotropic Gaussian kernel (full width at half maximum [FWHM] = 8 mm). ALFF Analysis The ALFF analysis was carried out using the software of REST (http://resting-fmri.sourceforge.net). First, the time series was transformed to a frequency domain with a fast Fourier transform, and the power spectrum was obtained. Since the power of a given frequency is proportional to the square of the frequency component amplitude of the original t time-domain series, the square root was calculated at each frequency of the power spectrum, and the average square root was obtained across low frequencies (0.01-0.08 Hz) at each voxel. This average square root was taken as the ALFF. For standardization purposes, the ALFF of each voxel was divided by the global mean ALFF value (25,26). Statistical Analysis The ALFF result with 1-sample t test was not demonstrative since it reflected all the brain regions with high LFF in addition to the default mode (26). Subsequently, the ALFF values of each group were analyzed using 2-sample t tests in SPM2, to compare the differences of the whole brain between each group of patients and controls. A statistical threshold of P , 0.001 (uncorrected) was used for an exploratory whole-brain analysis. RESULTS Within the 2-sample t test comparisons for the ALFF between BEB patients and controls, the patients showed increases in the ALFF within the left putamen, insular lobe, pallidum, and mPFC (Fig. 1; Table 1). The cerebellum, thalamus, posterior central gyrus, and the PCC showed decreased ALFF (Fig. 2; Table 2). 236 Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. DISCUSSION In our study, we found significant ALFF differences between BEB patients and normal controls. Some areas of the brain showed increased ALFF in the BEB group, including the left putamen, insular lobe, pallidum, and mPFC. Areas showing decreased ALFF in BEB included the cerebellum, thalami, medial and PCC, and somatosensory regions (Figs. 1, 2). Our results are consistent with some, but not all, previous findings in BEB studies. Biswal et al (20) found that ALFF was higher in the gray matter than in the white matter. In addition, Kiviniemi et al (27) reported activation in the visual cortex because of LFFs at approximately 0.034 Hz using the power spectrum method, indicating that ALFF may detect regional spontaneous neuronal activity. These studies employed regional homogeneity (ReHo) method (28) and functional connectivity analysis (29), tech-niques quite different from our ALFF method. ReHo and functional connectivity analyses focus on the similarities of intraregional and interregional time series, respectively, while ALFF measures the amplitude of regional activity. Fox and Raichle (10) have pointed out that activity in the DMN is highest during rest and reduced during cogni-tive activity. In all subjects, it comprises a large frontal area including vACC, mPFC, orbitofrontal cortex, PCC, IPL, and a temporal region involving the parahippocampal gyrus. In the present study, we found a significantly increased ALFF in mPFC and a decreased ALFF in the PCC of BEB patients. This suggests that there were distinct differ-ences in the DMN in BEB patients compared with normal controls. There is growing evidence that both functional and structural changes in the sensory cortex of patients with primary dystonia play pivotal roles in the pathophysiology of this disorder. The severity of dystonia correlated with the amount of overactivity in the primary somatosensory cortex (SI) during dystonic movements in patients with writer's cramp (30). Bilateral postcentral gyrus overactivity during whistling may be a functional correlate of altered somato-sensory representation in patients with orofacial dystonia (31). MRI voxel-based morphometry disclosed an increase of gray matter indicative of structural remodeling in primary somatosensory areas of patients with focal hand dystonia (32). In the present study, we found significantly decreased activity in the somatosensory region during the resting state in BEB patients compared to controls. Whether fMRI showed postcentral hypoactivity in the resting state, as we demonstrated, or hyperactivity during orofacial motor exe-cution, as reported by Dresel et al (31), the results indicate that the somatosensory cortex is involved in orofacial dys-tonia. The sensory trick, a so-called geste antagonistique TABLE 1. Areas showing increased amplitude of low-frequency fluctuation in comparing BEB patients and normal controls Anatomical Region Hemisphere MNI Coordinates (x, y, z) Brodmann's Area Cluster Size T Value Putamen L 215, 6, 26 48 19 4.62 Pallidum L 29, 3, 26 25, 48 2 4.63 Insular lobe L 233, 21, 12 47, 48 45 5.46 Superior frontal L 218, 6, 54 8, 9, 10 112 8.33 R 18, 3, 51 8, 9, 10, 11 106 5.75 Superior frontal, medial L 0, 42, 39 8, 9, 10 44 4.86 R 6, 30, 45 8, 9, 10, 32 93 5.86 L, left hemisphere; R, right hemisphere; MNI, Montreal Neurological Institute echo-planar imaging. FIG. 1. Comparison of the amplitude of low-frequency fluctuations (ALFF) between BEB patients and controls. The BEB patients showed increases in the ALFF within the left putamen, insular lobus, pallidum, and medial prefrontal cortex. Z, level of section in the axial plane; L, left hemisphere; R, right hemisphere. Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 237 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. such as touching or stroking the face periorbitally, can alle-viate the symptoms of patients with blepharospasm (33) and even transiently modulate the blink reflex circuitry (34). These findings are further evidence that the somato-sensory system is involved in the pathophysiology of this disorder. Our study showed an increased ALFF in the left putamen and pallidum and decreased ALFF in bilateral thalami in the BEB group. A majority of the studies on BEB and other dystonias have demonstrated abnormal metabo-lism of both the thalamus and basal ganglia (35-38), con-sistent with our findings. There are 2 pathways in the motor circuit of basal ganglia. The striatum sends inhibitory projections to the basal ganglia output nuclei (globus pallidus interna/substantia nigra, GPi/ SNr), which is called the direct pathway. It also sends inhibitory projections to the globus pallidus externa (GPe), which in turn inhibits the subthalamic nucleus (STN) and GPi. The latter indirect pathway from the striatum through the GPe and STN functions in an opposite manner to the direct pathway. The output from the basal ganglia (GPi/SNr) is inhibitory and projects to motor and premotor cortices, brainstem, and thalamus (39). The basal ganglia play a role in modulating lid movements through the pallidal-thalamic and nigral-collicular output pathways. The inhibitory projection of the globus pallidus to the thalamus can significantly modulate cortical activity associated with blinking. Many reports support the hypothesis that disturbances in striatal control of the globus pallidus (and substantia nigra pars re-ticulata) may be responsible for the altered neuronal activity in dystonia (2,40-42). It has been proposed that an increased activity in the direct striatopallidal pathway inhibits the inter-nal segment of the globus pallidus, resulting in an increased inhibitory synaptic activity in the medial globus pallidus and a reduced activity in pallidal output to the thalamus (1). Using fMRI, Schmidt et al (6) reported that a subregion of the putamen was active during spasms in BEB patients but not active during voluntary blinks in normal subjects. Blax-ton et al (43) thought that putamen activation had only been implicated in reflex eye blinks to air puffs and did not observe any striatal activation with voluntary eyelid movements. Schmidt et al (6) noted that unilateral or bilateral activation of the putamen correlated with eyelid spasms in 6 patients with BEB; these activations were reproducible after 2 years, although putamen activation was not observed during vol-untary blinking in any of the control subjects. Thus, altered putamen function may be a critical component of BEB. However, Suzuki et al (44) examined 25 patients with BEB following botulinum-A toxin injection using PET with 18-fluorodeoxyglucose (FDG). They found hyperactivity in the thalamus correlating with a trend of glucose hyperme-tabolism bilaterally in the putamen; yet there was no TABLE 2. Areas showing decreased amplitude of low-frequency fluctuation in comparing BEB patients and normal controls Anatomical Region Hemisphere MNI Coordinates (x, y, z) Brodmann's Area Cluster Size T Value Thalamus L 29, 212, 9 - 17 23.84 R 6, 218, 3 - 26 23.97 Cerebellum 6 L 230, 260, 230 - 30 24.52 Cerebellum crus 1 R 51, 269, 236 - 17 23.91 Precuneus L/R 26, 260, 69 5, 7 30 23.99 Post-/precentral gyrus L 251, 29, 26 3, 4 18 23.74 R 57, 212, 45 3, 4 10 23.91 L, left hemisphere; R, right hemisphere; MNI, Montreal Neurological Institute echo-planar imaging. FIG. 2. Comparison of the amplitude of low-frequency fluctuation (ALFF) between BEB patients and controls. The BEB patients showed decreases in the ALFF within the cerebellum, thalamus, posterior central gryus, and the posterior cingulate cortex. Z, level of section in axial plane; L, left hemisphere; R, right hemisphere. 238 Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. significant difference in comparison to controls (P , 0.01, uncorrected). The hyperactivity in the thalamus was also observed with the depletion of sensory feedback. Suzuki et al (44) concluded that thalamic hyperactivation may be one of the primary causes of BEB, as hyperactivity of the striatum is sensory input dependent. Also using PET, Kerrison et al (45) found decreased glucose uptake in the thalamus of patients with BEB, consistent with the results of the present study of decreased thalamic ALFF. Possibly, thalamic dysfunction is the primary defect in BEB. This is supported by the reports of blepharospasm in association with bilateral thalamic infarcts (46) and unilateral blepha-rospasm caused by an ipsilateral thalamomesencephalic hemorrhage (47). MRI and single-positron emission com-puted tomography demonstrated lesions of the basal gan-glia, the thalamus, or both in 5 of 7 patients with Meige syndrome (37), suggesting that voluntary motor control and reciprocal activity in the basal ganglia and thalamocortical circuits are impaired. A study by Esmaeli-Gutstein et al (2) employing PET with 18-FDG displayed striatal and tha-lamic hyperactivity in patients with BEB. This enhanced glucose metabolism reflected either increased excitatory or inhibitory neuronal activity in these regions. Further inves-tigation is required to clarify the different roles of the thal-amus and striatum in the pathophysiology of BEB. Our study showed decreased ALFF in the cerebellum of BEB patients, which is consistent with the previous findings in BEB patients (45). However, Suzuki et al (44) found significant hypermetabolism in the cerebellum of botuli-num toxin incomplete suppression BEB patients. Hutchin-son et al (5) reported with PET imaging that BEB patients exhibit hypermetabolism of the cerebellum and pons during wakefulness but not during sleep. Ceballos-Baumann et al (48) found that in patients with writer's cramp, cerebellar vermis activation was present before botulinum toxin administration but disappeared after the treatment. The cerebellum receives extensive somatosensory input via spi-nocerebellar pathways and processes sensory input (49). The abnormal metabolism in the cerebellum appears to be a secondary phenomenon related to muscular activity of the eyelids (6), probably not specific for BEB. There are a number of limitations to our study. First, it is difficult to ask participants lying in a machine creating a good deal of noise to close their eyes with thinking of nothing. Second, the small sample size limits the general-ization of our results. Third, 2 of our BEB patients had been treated with botulinum injections. Although the injections occurred 6 months before fMRI examination, it cannot be assumed that botulinum injections did not affect the cortical and subcortical BOLD signals. In conclusion, the resting state fMRI in BEB patients revealed deficient ALFF of the cerebellum, thalami, somatosensory region, and PCC and increased ALFF of the left putamen, pallidum, and mPFC. This suggests that an abnormal DMN and corticostriatopallidothalamic loop may be associated with the pathophysiology of BEB. Evaluating BEB patients with electromyography during fMRI could lead to better model functions and determine both the role and activation sequence of cortical and other subcortical areas in the neural network underlying BEB. REFERENCES 1. Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195-1212. 2. Esmaeli-Gutstein B, Nahmias C, Thompson M, Kazdan M, Harvey J. Positron emission tomography in patients with benign essential blepharospasm. Ophthal Plast Reconstr Surg. 1999;15:23-27. 3. Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee- Minnich L, Jankovic J, Moerlein SM. Decreased [18F] spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843-850. 4. Federico F, Simone IL, Lucivero V, Defazio G, De Salvia R, Mezzapesa DM, Petruzzellis M, Tortorella C, Livrea P. Proton magnetic resonance spectroscopy in primary blepharospasm. Neurology. 1998;51:892-895. 5. Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55:673-677. 6. Schmidt KE, Linden DE, Goebel R, Zanella FE, Lanfermann H, Zubcov AA. Striatal activation during blepharospasm revealed by fMRI. Neurology. 2003;60:1738-1743. 7. Baker RS, Andersen AH, Morecraft RJ, Smith CD. A functional magnetic resonance imaging study in patients with benign essential blepharospasm. J Neuroophthalmol. 2003;23,11-15. 8. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676-682. 9. Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685-694. 10. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700-711. 11. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1-38. 12. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253-258. 13. Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637-4642. 14. Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15-29. 15. Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836-2845. 16. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673-9678. 17. Goldman RI, Stern JM, Engel J Jr, Cohen MS. Simultaneous EEG and fMRI of the alpharhythm. Neuroreport. 2002;20:2487-2492. 18. Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A. 2007;104:18265-18269. Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 239 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 19. Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170-13175. 20. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537-541. 21. Yang H, Wu QZ, Buo LT, Li OQ, Long XY, Huang XQ, Chan RC, Gong QY. Abnormal spontaneous brain activity in medication-naive ADHD children: a resting state fMRI study. Neurosci Lett. 2011;502:89-93. 22. He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488-500. 23. Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, Tian L, Li Z, Shi J, Liu Y. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp. 2010;31:1851-1861. 24. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97-113. 25. Yang H, Long XY, Yang YH, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144-152. 26. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain. 2007;29:83-91. 27. Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magn Reson Med. 2000;44:373-378. 28. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394-400. 29. Tian LX, Jiang TZ, Wang YF, Zang YF, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2006;400:39-43. 30. Lerner A, Shill H, Hanakawa T, Bushara K, Goldfine A, Hallett M. Regional cerebral blood flow correlates of the severity of writer's cramp symptoms. Neuroimage. 2004;21:904-913. 31. Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos- Baumann AO. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain. 2006;129:36-46. 32. Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736-739. 33. Hallett M. Blepharospasm: recent advances. Neurology. 2002;59:1306-1312. 34. Gómez-Wong E, Martí MJ, Cossu G, Fabregat N, Tolosa ES, Valls-Solé J. The ‘geste antagonistique' induces transient modulation of the blink reflex in human patients with blepharospasm. Neurosci Lett. 1998;251:125-128. 35. Galardi G, Perani D, Grassi F, Bressi S, Amadio S, Antoni M, Comi GC, Canal N, Fazio F. Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand. 1996;94:172-176. 36. Macia F, Escola L, Guehl D, Michelet T, Bioulac B, Burbaud P. Neuronal activity in the monkey motor thalamus during bicuculline-induced dystonia. Eur J Neurosci. 2002;15:1353-1362. 37. Sakai T, Shikishima K, Kawai K, Kitahara K. Meige's syndrome associated with basal ganglia and thalamic functional disorders. Nippon Ganka Gakkai Zasshi. 1998;102:764-770. 38. Piazza P, Bettoni L, Bortone E, Cusumano F. MR findings in Meige syndrome. J Comput Assist Tomogr. 1989;13:116-118. 39. Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381-425. 40. Schicatano EJ, Basso MA, Evinger C. Animal model explains the origins of the cranial dystonia benign essential blepharospasm. J Neurophysiol. 1997;77:2842-2846. 41. Schneider S, Feifel E, Ott D, Schumacher M, Lu¨cking CH, Deuschl G. Prolonged MRI T2 times of the lentiform nucleus in idiopathic cervical dystonia. Neurology. 1994;44:846-850. 42. Colosimo C, Pantano P, Calistri V, Totaro P, Fabbrini G, Berardelli A. Diffusion tensor imaging in primary cervical dystonia. J Neurol Neurosurg Psychiatry. 2005;76:1591-1593. 43. Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JF. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J Neurosci. 1996;16:4032- 4040. 44. Suzuki Y, Mizoguchi S, Kiyosawa M, Mochizuki M, Ishiwata K, Ishii K Glucose hypermetabolism in the thalamus of patients with essential blepharospasm. J Neurol. 2007;254:890-896. 45. Kerrison JB, Lancaster JL, Zamarripa FE, Richardson LA, Morrison JC, Holck DE, Andreason KW, Blaydon SM, Fox PT. Positron emission tomography scanning in essential blepharospasm. Am J Ophthalmol. 2003;5:846-852. 46. Miranda M, Millar A. Blepharospasm associated with bilateral infarcts confined to the thalamus: case report. Mov Disord. 1998;13:616-617. 47. Kulisevsky J, Avila A, Roig C, Escartin A. Unilateral blepharospasm stemming from a thalamomesencephalic lesion. Mov Disord. 1993;8:239-240. 48. Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer's cramp. A PET study. Brain. 1997;120:571-580. 49. Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545-547. 240 Zhou et al: J Neuro-Ophthalmol 2013; 33: 235-240 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |