| Title |
Homonymous Hemianopia From Infarction of the Optic Tract and Lateral Geniculate Nucleus in Deep Cerebral Venous Thrombosis |
| Creator |
Grabe, Hilary M; Bapuraj, J Rajiv; Wesolowski, Jeffrey R; Parmar, Hemant; Jonathan Daniel Trobe MD, Michigan University |
| Affiliation |
Departments of Ophthalmology and Visual Sciences (HMG, JDT), Radiology (JRB, JRW, HP), and Neurology (JDT), University of Michigan, Ann Arbor, Michigan |
| Abstract |
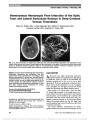
A 20-year-old man developed right homonymous hemianopia, hemiparesis, and hemisensory loss from deep cerebral venous thrombosis in the setting of high altitude. Approximately 3 months later, brain MRI showed encephalomalacia of the left optic tract and lateral geniculate nucleus, as well as signal abnormalities of the internal capsule and posterolateral thalamus. Homonymous hemianopia has previously been described in 1 case after deep cerebral venous thrombosis but without detailed neuroimaging features., (C) 2012 Lippincott Williams & Wilkins, Inc. |
| Subject |
Hemianopsia; Paresis; Encephalomalacia; Sinus Thrombosis, Intracranial |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227277 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69d03hd/227277 |