| Title |
Sellar and Parasellar Intravascular Lymphoma Mimicking Pituitary Apoplexy |
| Creator |
Rizek, Philippe; Seitelbach, Maayan; Alturkustani, Murad; Leung, Andrew; Fraser, J. Alexander |
| Affiliation |
Departments of Clinical Neurological Sciences (PR, JAF), Medicine (MS), Pathology (MA), Radiology (AL), and Ophthalmology (JAF), Schulich School of Medicine and Dentistry, University of Western Ontario, London, Ontario, Canada |
| Abstract |
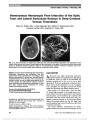
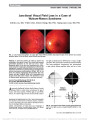
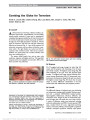
Intravascular lymphoma (IVL) is a rare subtype of large-cell non-Hodgkin lymphoma, characterized by proliferation of lymphoma cells within the lumina of small vessels. There are no previously reported cases of IVL involving the pituitary gland presenting with neuro-ophthalmic findings., Methods: A 68-year-old female presented with headache, right third nerve palsy, and Horner syndrome. MRI showed a 1.4-cm sellar mass consistent with a pituitary macroadenoma. Two weeks later, despite treatment with dexamethasone, the patient developed complete bilateral ophthalmoplegia and ptosis. Repeat MRI showed invasion of the clivus and cavernous sinuses, and a transsphenoidal pituitary biopsy was undertaken., Results: The preliminary histopathology was consistent with bland pituitary apoplexy, but subsequent examination of an incidentally biopsied nasal polyp revealed endovascular malignant lymphoid cells that, on further scrutiny, were also present in the pituitary tissue. The diagnosis of IVL was confirmed, and the patient had an excellent clinical and radiological response to cyclophosphamide, doxorubicin, vincristine, prednisolone, and rituximab (CHOP-R) chemotherapy., Conclusion: IVL may involve the pituitary gland, causing sellar mass effect, cavernous sinus infiltration, and pituitary ischemia, mimicking pituitary apoplexy with neuro-ophthalmic features. It can be effectively treated with CHOP-R chemotherapy., (C) 2012 Lippincott Williams & Wilkins, Inc. |
| Subject |
Lymphoma, Non-Hodgkin; Oculomotor Nerve Diseases; Horner Syndrome; Pituitary Neoplasms; Ophthalmoplegia; Blepharoptosis; Pituitary Apoplexy |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227276 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69d03hd/227276 |