| Title |
Visual Sequelae After Consensus-Based Treatment of Ophthalmic Artery Segment Aneurysms |
| Creator |
Kanagalingam, Sivashakthi; Gailloud, Philippe; Tamargo, Rafael J; Prem S Subramanian, MD, PhD, Professor of Ophthalmology, Neurology, and Neurosurgery, University of Colorado; Miller, Neil R |
| Affiliation |
Departments of Ophthalmology (SK, PSS, NRM), Radiology (PG), and Neurosurgery (RJT, PSS, NRM), The Johns Hopkins Hospital, Baltimore, Maryland |
| Abstract |
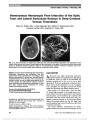
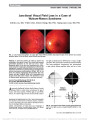
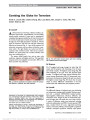
To determine the anatomic and visual outcomes of patients with ophthalmic artery segment aneurysms treated at The Johns Hopkins Hospital using a consensus-based treatment algorithm., Methods: Retrospective record review of a prospectively accrued case series of 88 patients (101 aneurysms) treated between January 2004 and July 2009. Presenting symptoms and aneurysm parameters were recorded for all subjects. Treatment strategy for all patients was determined by consensus among neurosurgeons, neurointerventionalists, neurologists, and neuroophthalmologists meeting to review the clinical cases on a weekly basis. Final clinical outcomes (aneurysm control, functional status, and vision) were ascertained from in-house examinations, medical records, telephone interviews, or a combination of these methods. Risk factors for visual or other complications were evaluated., Results: An optic neuropathy was present in at least 30 (34%) of 88 patients after treatment. Presumed new visual loss occurred in 24 (27%) of these patients. The remaining 6 patients had preexisting optic neuropathy-related visual loss that worsened after treatment. No patient with a preexisting optic neuropathy improved following treatment., Conclusion: Ophthalmic artery segment aneurysms present a treatment challenge because of their anatomic complexity and relationship to critical neural structures, particularly the visual sensory pathway. We have adopted a consensus-based treatment approach in an effort to optimize patient outcomes and aneurysm control. Although our approach resulted in durable treatment of the aneurysm, a sizable proportion of patients experienced new vision loss after treatment, and no patient with preexisting visual loss related to their aneurysm experienced visual improvement after treatment. We recommend that all patients with ophthalmic artery aneurysms receive careful and thorough preprocedural counseling to ensure they are aware of the risks and benefits of treatment regardless of the method used., (C) 2012 Lippincott Williams & Wilkins, Inc. |
| Subject |
Ophthalmic Artery; Aneurysm; Microsurgery; Endovascular Procedures |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227275 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69d03hd/227275 |