| Title |
Is Leber Hereditary Optic Neuropathy Treatable- Encouraging Results With Idebenone in Prospective and Retrospective Trials |
| Creator |
Sabet-Peyman, Esfandiar J; Khaderi, Khizer R; Sadun, Alfredo A |
| Affiliation |
Department of Ophthalmology (EJS-P, KRK, AAS), Doheny Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, California |
| Abstract |
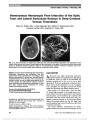
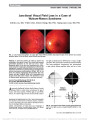
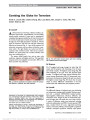
A 31-year-old woman developed subacute bilateral visual loss over a 2-week period. Two months later, the diagnosis of Leber hereditary optic neuropathy (LHON) 11778/ND4 was established and the patient was treated with 900 mg of idebenone daily. Over the ensuing 9 months, visual acuity improved from 20/200 to 20/25 in each eye with near-total resolution in visual field abnormalities. Our case report is in agreement with 2 large published series of patients with LHON treated with idebenone, raising hope for treatment of this visually devastating mitochondrial disorder., (C) 2012 Lippincott Williams & Wilkins, Inc. |
| Subject |
Optic Atrophy, Hereditary, Leber; Idebenone |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227282 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69d03hd/227282 |