| Title |
Reappraisal of the Optic Nerve Hypoplasia Syndrome |
| Creator |
Mark S. Borchert, MD, University of Southern California |
| Affiliation |
Keck Medical Center of the University of Southern California |
| Abstract |
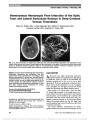
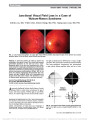
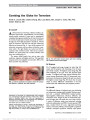
Optic nerve hypoplasia (ONH) has been described as an increasingly prevalent cause of congenital blindness. Its association with hypopituitarism and absent septum pellucidum has been recognized for more than 40 years as "septo-optic dysplasia" or "de Morsier syndrome." More recent studies have suggested that these associations are independent of one another. This review was designed to assess the historical and recent evidence for associations of neuroradiologic, endocrinologic, and developmental problems in patients with ONH., Evidence acquisition: Historical and contemporary literature review., Results: The medical literature does not support the notion that Georges de Morsier ever described a case of ONH or recognized its association with hypopituitarism or missing septum pellucidum. Recognition of the critical association of ONH with hypopituitarism should be attributed to William Hoyt. Hypopituitarism and other more recently identified associations with ONH, such as developmental delay, hypothalamic dysfunction, and autism, are independent of septum pellucidum development. Other common neuroradiographic associations, such as corpus callosum hypoplasia, gyrus dysplasia, and cortical heterotopia, may have prognostic significance., Conclusions: Children with ONH need to be monitored for many systemic, developmental, and even life-threatening problems independent of the status of the septum pellucidum. "Septo-optic dysplasia" and "de Morsier syndrome" are historically inaccurate and clinically misleading terms that should be abandoned., (C) 2012 Lippincott Williams & Wilkins, Inc. |
| Subject |
Septo-Optic Dysplasia; Hypopituitarism; Autistic Disorder; Hypothalamic Diseases; Septum Pellucidum |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227283 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s69d03hd/227283 |