Contents | 24 of 31
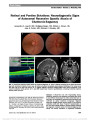
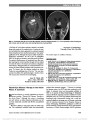
Localizing the Lesion in Foster Kennedy Syndrome From Hemiretinal Atrophy at the Macula
| Title | Journal of Neuro-Ophthalmology, December 2014, Volume 34, Issue 4 |
| Date | 2014-12 |
| Language | eng |
| Format | application/pdf |
| Type | Text |
| Publication Type | Journal Article |
| Collection | Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher | Lippincott, Williams & Wilkins |
| Holding Institution | Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management | © North American Neuro-Ophthalmology Society |
| ARK | ark:/87278/s6fv1s4v |
| Setname | ehsl_novel_jno |
| ID | 227666 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s6fv1s4v |
Page Metadata
| Title | Localizing the Lesion in Foster Kennedy Syndrome From Hemiretinal Atrophy at the Macula |
| Creator | Vislisel, Jesse M; Chen, John J; Kardon, Randy H |
| Subject | Humans; Male; Meningeal Neoplasms; Meningioma; Optic Nerve; Papilledema |
| Format | application/pdf |
| Publication Type | Journal Article |
| Collection | Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher | Lippincott, Williams & Wilkins |
| Holding Institution | Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management | © North American Neuro-Ophthalmology Society |
| Setname | ehsl_novel_jno |
| ID | 227658 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s6fv1s4v/227658 |