| Title |
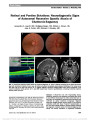
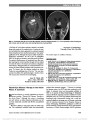
Isolated Optic Nerve, Chiasm, and Tract Involvement in BingNeel Syndrome |
| Creator |
Hughes, Michael S; Atkins, Edwards J; Cestari, Dean M; Stacy, Rebecca C; Hochberg, Fred |
| Subject |
Older people; Antineoplastic Agents, Hormonal; Dexamethasone; Female; Humans; Immunosuppressive Agents; Magnetic Resonance Imaging; Male; Methotrexate; Middle Older people; Optic Chiasm; Optic Nerve; Optic Tract ;Vision Disorders; Waldenstrom Macroglobulinemia |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227640 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6fv1s4v/227640 |