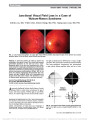
| OCR Text |
Show Shedding Light on Photophobia Kathleen B. Digre, MD, K.C. Brennan, MD Abstract: Photophobia is a common yet debilitating symp-tom seen in many ophthalmic and neurologic disorders. Despite its prevalence, it is poorly understood and difficult to treat. However, the past few years have seen significant advances in our understanding of this symptom. We review the clinical characteristics and disorders associated with photophobia, discuss the anatomy and physiology of this phenomenon, and conclude with a practical approach to diagnosis and treatment. Journal of Neuro-Ophthalmology 2012;32:68-81 doi: 10.1097/WNO.0b013e3182474548 © 2012 by North American Neuro-Ophthalmology Society Photophobia is reported in most all forms of migraine and many neuro-ophthalmic disorders. The symptom is a hallmark of primary eye conditions, such as uveitis, and certain retinal dystrophies. It is included as one of the major criteria for migraine in the International Classification of Headache Disorders (1,2). It is listed as a major symptom in blepharospasm. Yet in other clinical settings, some have suggested that photophobia is merely a functional symptom without an organic basis (3). NOMENCLATURE OF PHOTOPHOBIA The term photophobia is a misnomer and not quite accurate. It comes from 2 Greek words: photo - "light" and phobia - "fear or dread of"-hence, "fear of light." It is defined as an "abnormal sensitivity to light, especially of the eyes" (4). In defining photophobia, nearly 8 decades ago, Lebensohn (5) wrote, "exposure of the eye to light definitely induces or exacerbates pain." There are other terms and concepts of light aversion that must be distinguished from photophobia. Cummings and Gittinger (6) described "central dazzle" as an uncomfort-able, but not painful, sense of excessive brightness. They thought this represented a thalamic dysesthetic or hyper-pathia syndrome. Loewenfeld (7) described "the dazzling syndrome" as abnormal light scatter without ocular adapta-tion. "Hemeralopia" or "day blindness" refers to blurring of vision due to light and is a frequent complaint in patients with retinal (e.g., cone dystrophy) and rarely optic nerve disorders. In these cases, patients report they see better in dim illumination (8). We have used the term "photo-oculodynia" to describe pain or discomfort in the eye from a light source that is not usually painful (9). This term is in keeping with the literature that describes pain from a nor-mally nonpainful stimulus (e.g., cutaneous allodynia) (10,11). In this review, we define photophobia broadly as a sensory state in which light causes discomfort in the eye or head; it may also cause an avoidance reaction without overt pain. We use photo-oculodynia to describe light-induced eye pain from a normally nonpainful source (e.g., ambient lighting). CONDITIONS ASSOCIATED WITH PHOTOPHOBIA We performed a chart review of 111 adults (53 men and 58 women) and 36 children who were diagnosed in an eye clinic with "photophobia" (12) (Table 1). A cause was found in the majority of adults, while a diagnosis could not be found in most of the children. One half of the adults were unemployed, and about 25% felt that the symptom greatly affected their quality of life. Their most common ocular condition was dry eyes, while their most common neurologic disorder was migraine. Other neurologic condi-tions included depression, blepharospasm, and progressive supranuclear palsy (PSP). Departments of Ophthalmology (KBD) and Neurology (KBD, KCB), University of Utah, Salt Lake City, Utah. K. B. Digre was supported in part by an unrestricted grant from the Research to Prevent Blindness, Inc, New York, New York, to the Department of Ophthalmology and Visual Sciences, University of Utah. K.C. Brennan was supported by the National Institutes of Health (NS059072, NS070084) and the National Headache Foundation. The authors report no conflicts of interest. Address correspondence to: Kathleen B. Digre, MD, Departments of Ophthalmology and Neurology, University of Utah, 65 Mario Capecchi Drive, Salt Lake City, UT 84132; E-mail: Kathleen.digre@ hsc.utah.edu; K.C. Brennan, MD, Department of Neurology, University of Utah, 383 Colorow Drive, Salt Lake City, UT 84108; E-mail: K.C.Brennan@hsc.utah.edu. 68 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Section Editors: Grant T. Liu, MD Randy H. Kardon, MD, PhD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. TABLE 1. Conditions associated with photophobia Ocular Anterior segment Ocular inflammation (iritis, uveitis) Conjunctivitis Corneal diseases (corneal neuropathy, interstitial keratitis) Blepharitis Corneal neuropathy (15) Bilateral acute iris transillumination defects of the iris (137) Dry eyes: the most common cause of photophobia (including Grave orbitopathy (138)) Pterygium (139): most common ocular cause Corneal neuropathy Interstitial keratitis (Cogan syndrome) Posterior segment Vitritis Uveitis Photoreceptor dysfunction/retinal dystrophy (16) Albinism, achromatopsia, cone dystrophy, retinitis pigmentosa Alström syndrome (19) Sjogren-Larsson syndrome (140) Retinal dystrophy (16) Optic nerve Optic neuritis Papilledema Chiasm Pituitary tumor (including apoplexy) Hypophysitis (22) Thalamic pathology (tumor, stroke, hemorrhage) Occipital lobe Alteration in excitability (migraine) (113-115) Neurologic Migraine (the most common neurologic cause) Blepharospasm Progressive supranuclear palsy Traumatic brain injury Meningeal irritation (meningitis, subarachnoid hemorrhage) Psychiatric Agoraphobia Anxiety disorder (panic disorder) Depression Medications Barbiturates Benzodiazepines Chloroquine Methylphenidate Haloperidol Zoledronate (141) Other: Hangover headache Neurasthenia (chronic fatigue) Fibromyalgia Measles Rabies Inflammatory bowel disease IFAP syndrome (ichthyosis follicularis with alopecia and photophobia) PPK (psoriasiform lesions and palmoplantar keratoderma) (142,143) Trisomy 18 (144) Zinc deficiency with exocrine insufficiency (145) Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 69 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Anterior Segment Disease Anterior segment disease such as iritis, cyclitis, and blepharitis has long been known to cause photophobia. Lebensohn (5) found that the more superficial the corneal lesion, the more severe the photophobia. These disorders are presumably due to direct irritation of the trigeminal afferents that innervate the cornea and eye. Dry eyes and dry eye syndrome are a common ocular cause of photophobia (13). Data suggest that dry eyes can eventually lead to a corneal neuropathy that may persist after the dry eyes have cleared (14). Rosenthal (15) proposed that if a patient has symptoms of dry eyes and photophobia, but the examination does not support the diag-nosis of dry eyes, one should consider "corneal neuropathy until proven otherwise." Rosenthal used corneal biomicro-scopy to show changes in the structure of the corneal nerves of patients with dry eyes (15). Corneal neuropathy can be triggered by a variety of conditions including dry eyes, zoster keratitis, diabetic neuropathy, and chemotherapy (14,15). Posterior Segment Disease Posterior segment disease, such as retinal dystrophies, retinitis pigmentosa, and cone dystrophies, has been associ-ated with photophobia. At times, hemeralopia or frequent photopsias are the presenting symptoms. Prokofyeva et al (16) reported that in addition to changes in visual acuity and night vision, photophobia was a frequent early symptom of retinal dystrophies and cone disorders. Photophobia may be one of the earliest signs of cone dystrophy before visual loss, and the diagnosis of malingering frequently can be made (17,18). Patients with conditions such as Alström syndrome have photophobia from initial onset (19). It is appropriate to evaluate patients with photophobia for a retinal disorder if the cause of the photophobia is not apparent. If there are other visual symptoms such as visual loss, retinal disorders must be considered. Intracranial Conditions Intracranial conditions, such as meningeal irritation from meningitis (20), subarachnoid hemorrhage (21), or pituitary tumors or apoplexy (22) cause photophobia, are thought to be due to irritation of the basal meninges especially around the diaphragma sellae (3). This pain is mediated by branches of the first division trigeminal nerve, which innervates the meninges (23). Migraine Migraine is the most common neurologic disorder causing photophobia, and photophobia is one of the major di-agnostic criteria for migraine according to the International Classification of Headache Disorders (1,2). Up to 80% of migraine patients experience photophobia during an attack (24). The recent ID Migraine validation study suggested that the presence of photophobia, disability, and nausea predicted migraine approximately 98% of the time (25). Drummond and Woodhouse (26) showed that migrai-neurs were more light sensitive both during and between migraine attacks compared with nonmigraine controls. Vanagaite et al (27) reported that patients with migraine experience increased light sensitivity to progressively increased amounts of light during and between headache episodes compared with controls. They concluded that photophobia "seems to be an intrinsic property of migrai-neurs." Furthermore, 30%-60% of migraine attacks are triggered by light or glare (28). Different visual stimuli known to provoke migraine include sunlight, flickering from motion pictures, television, and fluorescent lights (28,29). It has been postulated that migraine is associated with "visual pathway dysfunction" from retina to occipital lobes (30). Migraine is not the only headache type associated with photophobia. Subjects with tension headache have more light sensitivity than controls (31). Unilateral photophobia has been reported with cluster headache, hemicrania con-tinua, and other trigeminal autonomic cephalalgias (32,33). In fact, the presence of unilateral photophobia is considered important in establishing the diagnosis of the trigeminal autonomic cephalalgias (32). Traumatic Brain Injury Traumatic brain injury (TBI) is commonly associated with photophobia. Acute TBI causes displacement, irritation, or injury of pain-sensitive intracranial structures, which likely accounts for both the headache and photophobia associated with brain injury (see below for possible pathophysiology). However, photophobia often remains after initial injury. There is an increased sensitivity to light in the subacute period (7-19 days) after head injury (34). Although most patients with mild head injury are improved after 6 months (35), those with postconcussive syndrome retain an inc-reased sensitivity to light (36). A large contribution to posttraumatic photophobia, especially chronic symptoms, may be due to the comor-bidity of migraine-like headache after TBI. In a meta-analysis of pain conditions after TBI, headache prevalence was 57.8% (95% confidence interval, 55.5, 60.2%) (37). A study examining affective symptoms (posttraumatic stress disorder and depression) after mild TBI also found an independent association of TBI with headache (38). However, these large studies did not use the International Classification of Headache Disorders criteria to define headache type, and thus, the photophobia component is difficult to ascertain. A smaller study of returning Iraq and Afghanistan veterans did use the International Classifica-tion of Headache Disorders criteria and found a greater likelihood of migraine (which has photophobia as a diag-nostic criterion) in veterans with more frequent injuries and abnormal findings on neurologic and neuropsycho-logic examinations (39). Clearly, these data are not con-clusive, and they speak to the need for more precise 70 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. ascertainment of both photophobia and headache symp-toms after TBI (40). Blepharospasm Blepharospasm is a focal dystonia associated with involun-tary blinking, squeezing, and closure of the eyelids. The cause is unknown but is thought to be due to an excitation/ inhibition imbalance in brainstem blink reflex pathways (41). While it has long been known that blepharospasm is associated with photophobia, our understanding of this association is limited. In one large survey of blepharospasm patients, Anderson et al (42) found that 80% reported aggravation of blepharospasm with bright lights while driv-ing, watching television, or reading. In a survey of 316 blepharospasm patients, 94% reported light sensitivity; ambient lighting could provoke spasms about half of the time, but bright light provoked spasms almost all of the time (43). We studied 30 subjects with blepharospasm, 30 patients with a known photophobic state (migraine), and 30 controls with no history of migraine or blepharo-spasm (44). We found that patients with blepharospasm were as light sensitive as patients with migraine and that both groups were more light sensitive than controls. Progressive Supranuclear Palsy PSP is associated with photophobia and may be quite specific for the disorder; one study showed that the symptom of photophobia alone differentiated PSP from corticobasal degeneration (45). In a comparative study of PSP and Parkinson disease, patients with PSP had photo-phobia more frequently (13 of 16 patients vs 6 of 14) (46). Psychiatric Conditions Photophobia has been reported in association with agora-phobia. Patients with agoraphobia frequently wear dark glasses and feel more relaxed in darkness. Light will frequently trigger anxiety reactions in these patients. In one study, illumination levels were normalized in agora-phobia patients who underwent successful cognitive behav-ioral therapy (47). Seasonal affective disorder is treated by viewing light (48), but little literature exists about photophobia and depression. One case report of a woman with bipolar depression revealed that she used eye patches during her depressive phase, and she used her light sensitivity to predict when she would be entering a depressive phase (49). Gerbaldo and Thaker (50) reported that photophobia occurs in patients with "neuras-thenia" (now known as chronic fatigue), bipolar depression, or seasonal depression. In contrast, patients with schizophre-nia are known to have "sun-gazing" tendencies without any discomfort (50). In a study of bright light therapy for depression, photophobia and other ocular symptoms also decreased with phototherapy. This initially counterintuitive response, decrease in light aversion with light therapy, sug-gests a powerful influence of affective circuits on photophobia (51). Patients with anxiety and panic disorder also tend to have a lowered threshold of tolerance to light. The light threshold in these patients also responded to behavioral mod-ifications and therapy (52). Given the comorbidity of these disorders with neuro-ophthalmic disease (especially mig-raine), one might expect an association. Clearly, more research is needed. IS PHOTOPHOBIA REALLY A NONORGANIC SYMPTOM? The "sunglasses sign" suggests factitious visual loss (53). Furthermore, photophobia is thought to accompany sec-ondary gain, and a review of cases for an independent visual examination discounted this symptom as factitious (54). For many years, migraine and blepharospasm were thought to be functional disorders (55). Certainly, photophobia accompanies depression and anxiety (52); this can make patient interaction and management difficult. As already discussed, photophobia can be diagnosed in association with migraine, blepharospasm, PSP, depression, and anxiety-all conditions with an anatomical and physiologic basis-and for which many treatments are now available. As will be seen below, photophobia has a rich and growing neurobi-ology. While photophobia can undoubtedly be associated with factitious disorders, it is unlikely to be a purely "psy-chiatric" symptom. ANATOMY AND PATHOPHYSIOLOGY OF PHOTOPHOBIA Trigeminal Nociceptive innervation Photophobia is intimately, likely inextricably, linked to pain sensation. The trigeminal nerve and its nuclei are the primary mediators of pain sensation to the head. The eye. Afferents from the ophthalmic (V1) portion of the trigeminal ganglion transmit pain information from the eye. The conjunctiva, cornea, sclera, and uvea (iris, ciliary body, and choroid) are densely innervated with trigeminal fibers and exquisitely sensitive to pain. Any painful stimulus to these areas (e.g., corneal abrasion, iritis, uveitis) invari-ably causes photophobia. In contrast, the retina is insensate, as evidenced by the lack of pain with retinal detachment and nonarteritic anterior ischemic neuropathy. The optic nerve does contain trigeminal afferents (not within the nerve, but within blood vessels and dura), which cause pain associated with optic neuritis and arteritic anterior ischemic neuropathy (56,57). The orbit. Other structures in the orbit are also pain sensitive. As would be expected, trigeminal V1 branches are nociceptive. Extraocular muscles have nociceptive affer-ents that travel along cranial nerves (CN) III, IV, and VI, leading to pain with orbital myositis. The pain of diabetic third nerve palsy is not clearly understood but is likely due Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 71 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. to nociceptive afferents within CN III or the extraocular muscles that it innervates. Orbital blood vessels are trigemi-nally innervated and contribute to the pain of orbital inflammation and superior orbital fissure syndrome (56,57). Autonomic Innervation The eye and orbit are densely innervated by autonomic effectors, most of which course along branches of the trigeminal nerve. Although not formally considered auto-nomic in their own right, trigeminal ganglion neurons are effectors as well as sensors. When activated by a nociceptive stimulus, they release mediators, including calcitonin gene- related peptide and nitric oxide from the very terminals that were activated. This positive feedback loop underlies the trigeminovascular reflex, whereby trigeminally innervated cranial vessels dilate after a nociceptive stimulus, perpetuat-ing nociceptor activation. The trigeminoautonomic reflex is a true multisynaptic reflex, involving activation of superior salivatory and Edinger-Westphal nuclei by collaterals from the trigeminal nucleus caudalis (TNC; the first sen-sory relay in the central nervous system mediating pain sensation from the head). Superior salivatory outputs ac-tivate parasympathetic effectors in the pterygopalatine ganglion (which dilate vessels) and in the ciliary ganglion (which mediate lacrimation). Edinger-Westphal outputs mediate pupillary constriction. The trigeminovascular and trigeminoautonomic reflexes are thought to underlie the conjunctival injection, tearing, and periorbital pain of migraine and cluster headache, which are almost invari-ably also accompanied by photophobia (58-61). The orbit is also densely innervated by sympathetic efferents. The short ciliary nerves carry sympathetic supply to the blood vessels in the orbit, and the long ciliary nerves supply sympathetic innervation to the pupil. The cornea also receives sympathetic innervation (62). Stimulation of the superior cervical ganglion in humans causes pain (63), and pharmacological blockade of this ganglion produces relief in patients with intractable facial pain who have failed trigeminal section (63). These findings are consistent with the understanding of sympathetic influences on somatic pain, best demonstrated in complex regional pain syndromes (CRPS; CRPS I: reflex sympathetic dystrophy, CRPS II: causalgia). Usual treatment of CRPS involves sympathetic block to the involved limb. We reasoned that sympathetic blockade might analogously reduce ocular pain and photophobia. Six patients with severe photophobia underwent placebo-controlled superior cervical ganglion blockade. All had marked reductions in spontaneous and light-triggered pain using local anesthesia but not with saline placebo (9). A second study with 19 patients showed similar findings (64). Blink Reflex The blink reflex is commonly considered as a response to stimulation of the cornea or face, but it is also induced by light, auditory stimuli, and distant somatosensory stimuli (65,66). Each stimulus-induced reflex has individual wiring features, although all have in common motor outflow through the seventh CN. The blink reflex is likely relevant to photophobia, as photophobia causes an increase in blink rate. Conversely, conditions associated with blinking abnor-malities (blepharospasm, PSP) also are associated with pho-tophobia (67). This 2-way interaction (photophobia leads to blinking; blinking leads to photophobia) suggests that there is cross talk between the different blink reflex path-ways, at least as regards the percept of photophobia. One likely location for this is the medullary laterobulbar reticular formation, which integrates sensory input prior to CN VII output (65). The corneal blink reflex begins with unmyelinated afferents from ciliary branches of V1, which synapse in the TNC. The supraorbital blink reflex involves mixed (myelin-ated and unmyelinated) afferents, which synapse in both TNC and the principal trigeminal nucleus. Blink reflex can also be elicited by stimulation of branches of the facial nerve; however, as it has a similar latency to trigeminally evoked responses, it likely involves a similar circuit. Surprisingly, little is known about the light-evoked blink reflex: it is assumed that visual pathways including retina, optic nerve, and olivary pretectal nucleus are involved (65,66). The light-evoked blink reflex is consensual and has a longer latency than other blink reflexes; evidence that it is multisynaptic. It is thought that tactile- or electrical-stimulation-induced blink reflex signals converge on the trigeminal dorsal horn and laterodorsal reticular formation. There are also direct connec-tions between principal nucleus of CN V and the olivary pretectal nucleus, directly to CN VII. The relatively long latency of the consensual blink response to supraorbital stim-ulation, and the even longer latency of the light-evoked blink response, suggests that these direct connections may not be involved (65,66,68,69). The circuitry of the blink reflex is very likely a part of the photophobia response given the increased blink rate in photophobic patients (70). Whether such circuit changes are causal or a consequence of some other pathology is unclear. Certainly, in the case of blepharospasm, abnor-malities in the blink reflex pathway could be of primary importance (41). Light Perception and Photophobia The cardinal stimulus for photophobia is light; thus, pathways of light perception must be involved. Interest-ingly, photophobia can be experienced without image formation, as documented in some blind patients (71-74). Rods and cones are the primary light sensors in the eye. They transmit photosignals via bipolar and amacrine cells to retinal ganglion cells, which exit the orbit via the optic nerve. Most of these fibers travel to the lateral geniculate nucleus of thalamus and then to occipital cortex, mediating vision. However, some travel to the olivary pretectal 72 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. nucleus, which via efferents in the Edinger-Westphal nucleus helps mediate pupillary constriction and accom-modation. Others travel to the suprachiasmatic nucleus to help entrain the circadian cycle (75-78). More recently, a separate set of photosensors has been identified (79-81). Termed intrinsically photosensitive ret-inal ganglion cells (IPRGCs), these contain the photopig-ment melanopsin rather than rhodopsin. IPRGCs both detect light (in a nonimage-forming manner) and project the photosignal to the olivary pretectal and suprachiasmatic nuclei. They are now considered more important than rods and cones for circadian photoentrainment (82,83). These cells are present in the retina (w1%-3% of ganglion cells) but surprisingly have also been identified in the iris (84). Thus, the eye may be photosensitive in ways that we have not even suspected until recently. Integration of Light Sensation With Nociceptive and Autonomic Responses in Photophobia It is intuitive that photophobia has elements of light perception and pain, but what are the neural circuit correlates of this multisensory experience? Photophobia circuits. Recent studies have begun to eluci-date the functional pathways that mediate photophobia. Two distinct circuits have been identified, and at least a third is possible. Given the redundant and parallel nature of homeostatic networks, it is likely that these networks are interconnected and interact with each other. It is also quite possible that more circuits remain to be discovered. Okamoto et al (85) recorded in the TNC as they shone light into the eyes of anesthetized rats. They found that the firing rate of TNC neurons increased on light exposure, a finding whose simplest interpretation is a nociceptive response to light (or photophobia). They could eliminate this light-evoked nociceptive discharge by lidocaine injec-tion into either the globe or the trigeminal ganglion, show-ing that both intraocular afferents and trigeminal neurons were required for the response. Next, via lidocaine injection into the superior salivatory nucleus (SSN) or injection of vasoconstrictors into the globe, they showed that parasym-pathetic efferents (likely causing ocular vasodilation) were involved. The circuit that emerges (Fig. 1) is of retinal photodetectors (whether rod, cone, or IPRGC is unclear) activating SSN, which in turn evokes ocular vasodilation and activation of pain-sensing neurons on blood vessels. Noseda et al (86) identified a completely different cir-cuit. By injecting a viral tracer into the globe, they made the surprising finding that a population of IPRGCs make direct connections in thalamic nuclei not normally associated with vision. These nuclei (posterior, lateral posterior, and inter-geniculate) are associated with somatosensation and pain. Next, they recorded from these retinally connected thalamic neurons and found that they responded to painful stimula-tion of the dura and to light. The convergent input from trigeminal and retinal afferents on the same thalamic neurons makes them uniquely positioned to interpret light as a nociceptive signal. In a sense, they can be con-sidered "photophobia neurons" (Fig. 1). Noseda et al fur-ther characterized the projection pattern of these thalamic neurons into cortex. They found processes in multiple regions, including visual, somatosensory, and association cortices. This projection pattern suggests a broadly dis-tributed, multisensory, and nociceptive response for photophobia. The role of the thalamus in this proposed multisensory integration bears mention. The thalamus is a critical state-setting and gain-setting region in the brain. Its role as a relay for sensory information is well understood, but the concept of a relay implies that little processing occurs, and this is not the case. Both gain and precision of sensory signals are actively altered in thalamus (87). Moreover, as the work of Noseda et al (86) demonstrates, there is integration of dif-ferent sensory pathways within the region. However, the role of the thalamus cannot be properly appreciated in iso-lation. There is massive reciprocal innervation between thal-amus and cortex, making it more physiologically realistic to consider sensory alterations as taking place in a "thalamo-cortical unit" (88). This has been best demonstrated in the study of sleep and waking state-associated oscillations (89), but it is surely equally relevant for sensory responses, which are themselves significantly modulated by state (90). Future research about thalamic roles in photophobia will hopefully uncover this sort of dynamic interaction. Photophobia without the optic nerve? It may be that pho-tophobia does not depend on any form of retinal photo-transduction. Dolgonos et al (91) measured trigeminal blink reflex by stimulating the supraorbital nerve in rats. They replicated the well-known finding that light potentiates (increases in amplitude) the trigeminal blink reflex. What was surprising was that this effect persisted after they sec-tioned the optic nerve, which should disconnect any photo-signal leaving the eye. The authors suggested that "associational ganglion cells" either directly activate trigem-inal nociceptors in the orbit or indirectly activate them through effects on uveal blood flow. Another possible explanation, given the recent discovery of melanopsin pho-toreceptors in the iris (84), is that these intrinsically photo-sensitive ganglion cells bypass the retina and optic nerve entirely to activate nociceptors either inside or outside the globe. Thus, there are at least 2 and possibly 3 ways that light from the eye can activate pain circuits: through conven-tional rod and cone circuits, through IPRGCs, and through melanopsin-containing ganglion-type cells outside the ret-ina. There are also at least 2 pathways by which light-based nociception can take place when exiting the globe, either through optic nerve projections to the pretectal nucleus or direct connections to the thalamus. It is also possible that intraocular trigeminal afferents are directly activated by extraretinal melanopsin-containing ganglion-like cells. Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 73 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. A caveat about the extraretinal pathway is that it may not be directly applicable in humans, as extraretinal melanopsin-containing cells have not been conclusively demonstrated. Although many conventional concepts of photophobia have been challenged in the past few years, it still appears that ocular contents are necessary, as complete enucleation removes the photophobia response (73,92). Possible molecular targets in photophobia. The intracranial nociception involved in migraine prominently involves activity of the calcium gene-related peptide (CGRP) recep-tor. As mentioned above, trigeminovascular afferents both release and respond to CGRP, which is both pro-nociceptive and vasodilatory. Because of its prominent role, CGRP is a target for acute migraine treatment, and CGRP antago-nists alleviate acute migraine (93,94). Interestingly, mice with gain-of-function mutations in the CGRP signaling pathway demonstrate symptoms consistent with migraine. The most prominent of these is photophobia (95,96). CGRP binds a heterodimeric receptor formed of the CRLR and RAMP1 proteins, activating adenylate cyclase and cyclic adenosine monophosphate. Recober et al (97) generated mice with increased sensitivity to CGRP via insertion of a human RAMP1 protein at high expression levels (nestin/hRAMP1 mice). These mice were no different from wild-type littermates in ocular anatomy, motor activity, or anxiety. However, they had a significant increase in light aversion on intracerebroventricular CGRP injection compared to the same littermates. This difference in light aversion was blocked by treatment with a CGRP antagonist, showing that the behavior was mediated by CGRP receptor activity. It will be very interesting to see what elements of the photophobia circuits are altered in RAMP1 mutant mice. Insights gained could lead to ana-tomically specific targets for migraine treatment (98). Photophobia circuit alterations in humans: Psychophysics of photophobia. It is clear that there are individual "thresholds" of light sensitivity even in normal people. Most studies that have looked at thresholds of light in individuals showed some normal variation (27). However, subjects with mig-raine and even tension-type and cervicogenic headache (27) have lower thresholds (27,99). Light sensitivity may also vary by season. Vanagaite et al found that migraine patients, and to a lesser extent controls, had lower pain thresholds to light in the winter months (November through January) FIG. 1. Photophobia circuits. 1. Ganglion cells project light-related signaling to the olivary pretectal nucleus (OPN; light green). OPN projections activate SSN (dark green), which via pterygopalatine ganglion, causing ocular vasodilation and ac-tivation of ocular trigeminal afferents (orange), which are heavily expressed on blood vessels. These afferents, with cell bodies in the trigeminal ganglion, project to TNC, thalamus, and cortex. 2. IPRGCs project directly to thalamic neurons (blue) that also receive intracranial nociceptive afferent signal (yellow neurons in trigeminal ganglion and TNC). Thalamic neurons fire in response to light and pain stimuli. Their output projects diffusely to sensory and association cortex. 3. Melanopsin-containing, intrinsically photosensitive ganglion-like cells have been identified in rodent iris. These afferents may explain the fact that light can activate trigeminal blink reflex even after the optic nerve (through which circuits 1 and 2 pass) has been sectioned. All 3 circuits may interact at different locations (created from references 84-86,91). 74 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. than in the summer months (May through July). There also appears to be a "summation" or "integration" involved in photophobia. Wirtschafter et al (100) showed that binocu-lar viewing lowered the threshold of light, whereas uniocular viewing raised the threshold. Finally, the perception of light brightness is dependent on the state of retinal adaptation (e.g., dark adaptation), as is readily appreciated when step-ping from a darkened movie theater into the sunshine (101,102). The wavelength of light may also affect the photophobia percept. Main et al (103) found that shorter wavelength (blue) light was more uncomfortable for subjects with migraine than for those with tension-type headache or con-trols. These investigators also reported that longer wave-length (red) light was also less comfortable for subjects with migraine (103). Good et al (29) found that visually provoked beta brain activity was suppressed by red light and enhanced with blue light in migraine patients, showing that the two wavelengths have different effects on cortical activ-ity. The reasons for this difference, and the noxious nature of both blue and red light to migraineurs, are unclear. The fact that intrinsically photosensitive retinal ganglion cells are preferentially sensitive to blue light is intriguing (83,104). Light stimulation can increase pain in the trigeminal and cervical regions in migraineurs compared with normal controls. Kowacs et al (105) performed pressure algometer readings over the head, before and after stimulation with light up to a discomfort threshold. All migraine subjects had reduced thresholds of light sensitivity, which was not unexpected, but they also had significant and sustained lowering of pressure and pain sensitivity in both trigeminal and cervical sites. Controls did not exhibit this phenomenon. Functional imaging of photophobia networks in humans. Functional neuroimaging allows the study of photophobia networks in awake humans. This is proving critical in con-firming knowledge gained in laboratory animals, but more importantly has allowed unique insights into the conscious perception of photophobia. Moulton et al (106) performed BOLD functional MRI (fMRI) recordings in an individual with photophobia asso-ciated with overuse of contact lenses. During the photopho-bic state, activation of the trigeminal ganglia, TNC, ventroposteromedial thalamus, and anterior cingulate gyrus occurred, but without photophobia, these structures were not activated. This pattern of activation suggests that pho-tophobia was perceived as a truly painful stimulus. Trigem-inal ganglion, TNC, and ventroposteromedial thalamus are all relays in cranial nociception, and the anterior cingulate cortex is an element in the "pain matrix," a network of cortical and subcortical structures activated during the con-scious perception of pain (107). In a series of patients with laser-assisted in situ keratomileusis-induced photophobia, Malecaze et al (108) found that light-induced BOLD fMRI activation was increased in the visual association cortex, compared with controls. This suggests that photophobia may involve alterations in cortical visual processing and pain networks (see below) (108). The photophobia of blepharospasm has also been investigated with functional imaging. Emoto et al (109) used 18-fluorodeoxyglucose positron emission tomography (PET) to compare blepharospasm patients with and without photophobia to controls. They found that blepharospasm patients with photophobia had significantly increased met-abolic activity in the thalamus and dorsal midbrain com-pared to nonphotophobic patients and controls. The regions with the largest differences between photophobic and nonphotophobic patients were thalamic ventral anterior (VA) and ventral lateral (VL) nuclei and the superior colli-culus. Interestingly, the nonphotophobic patients showed significantly decreased activity in the superior colliculus compared to controls. Both patient groups, by virtue of their blepharospasm, had increased rates of blinking com-pared to controls. VA, VL, and superior colliculus are all involved in motor control; thus, it is interesting that pho-tophobic and nonphotophobic patients-equally affected by the motor symptoms of blepharospasm-showed differ-ential activity patterns. However, superior colliculus is also involved in ocular-related sensorimotor integration; the dif-ference in its activity might be explained by greater noci-ceptor input in photophobic patients. Photophobia networks in migraine. Migraine is the most common clinical disorder associated with photophobia. A particular advantage of migraine for the study of photo-phobia is that (for most) the symptoms are intermittent. The migraine patient can thus serve as his or her own control. In addition to examining photophobia pathways in rodents (see above), Noseda and Burstein (73) examined humans with migraine-associated photophobia. These patients were unusual in that they all had specific visual pathway defects. Those who had undergone enucleation did not experience increased headache pain on exposure to light, but those with an intact eye did, confirming earlier clinical reports (70-74) and the laboratory work of Oka-moto et al (85), which showed that ocular afferents were necessary for photophobia. The presence of photophobia in legally blind patients supports (but does not confirm) the findings of Noseda et al (86) that IPRGCs, nonimage-form-ing ganglion cells, were responsible for the photophobia phenotype. Migraine is associated with alterations in cortical excitability (110), and visual aura can be directly correlated with occipital dysfunction (111,112). Denuelle et al (113) used 15O PET to examine the response to light during migraine, after treatment with sumatriptan, and interictally. They found that light levels that did not cause pain either interictally or after sumatriptan treatment evoked pain dur-ing headache, confirming migraine-induced photophobia. Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 75 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. They found that their photophobia stimulus induced a sig-nificant increase in cortical blood flow during the migraine attack (both before and after sumatriptan treatment) but not interictally. This shows that the occipital neurovascular response is more sensitive during attacks and is likely in-volved in the photophobia response (114,115). In a separate study, the same group examined migraine patients exclu-sively in the interictal period and demonstrated that even at "baseline," they had larger areas of occipital activation to visual stimulation, and visual stimulation paired with pain, compared to controls (114). Martin et al (116) showed similar findings using BOLD fMRI, with increased area of occipital activation to light during migraine attacks. Al-though there were some differences between the studies, their combined results show that the migraine-associated photophobia response persists into the interictal period, suggesting long-lasting alterations in sensory gain. The find-ings of increased response to pain + light, compared to light alone, are consistent with animal work showing multisen-sory integration during the photophobia response (86). APPROACH TO THE DIAGNOSIS OF PHOTOPHOBIA Before treating photophobia, one needs to be certain of the cause (Fig. 2). Careful history, along with neurologic and neuro-ophthalmic examination including visual fields, is essential. Pituitary tumor, meningitis, and other intracranial processes can present with photophobia. If there are focal neurologic findings, MRI of the brain is indicated. Other central causes, such as PSP, should be considered. However, the most common causes are dry eyes, "corneal neuropa-thy," and migraine. Diagnosing dry eye can be difficult. Examination techniques should include examination of the tear film, tear film breakup time, corneal staining with rose bengal or fluorescein, and Schirmer testing. Corneal neuropathy is also difficult to diagnose. We instill lidocaine eyedrops, and if the pain and discomfort resolve, this diagnosis is possible. Rosenthal (15) reports that corneal nerve imaging by confocal microscopy also may be helpful. Diagnosing migraine should not be a problem when one looks for pain associated with photophobia, phonophobia, nausea and/or vomiting, and pain that worsens with activity. Blepharospasm is usually not a challenge to diagnose if one observes frequent blinking. However, reflex blepharospasm in response to bright light can be difficult to identify. Look for squeezing of eyes, apraxia of eyelid opening, and involuntary eye closure to even moderate levels of light. Screening for depression and anxiety is also important. We conducted a study in which patients with chronic photophobia and migraine had increased scores on measures of depression and anxiety, compared to patients with episodic photophobia with migraine and to controls without migraine or photophobia (117). TREATMENT OF PHOTOPHOBIA There are few neuro-ophthalmic problems that can be as vexing to treat for clinicians. Are there really any known treatments? Certainly, there have been no major random-ized controlled trials of treatment of photophobia. Most of the literature consists of case reports, and a few studies with small numbers of subjects. Use and Misuse of Tinted Lenses One principal treatment is to decrease the dark-adapted state. Patients with severe photophobia who wear darkly tinted lenses should be encouraged to reduce dark adapta-tion. Chronic darkness will increase the perception and pain of light sensitivity. Lebensohn (5) cautioned that "tinted glasses as a symptomatic remedy for chronic photophobia are to be condemned because of both their ineffectiveness and their habit forming tendency." Wearing sunglasses to an eye clinic has led many physicians to consider the patient to have some type of psychiatric disorder or at least to pre-dict nonorganic visual loss (118,119). Some optical tints have been tried successfully to combat photophobia. Red-tinted contact lenses have been tested in individuals with photophobia due to cone disorders (120- 124). However, red tint appears to exacerbate migraine-associated photophobia (103). Sunglasses do make sense in the bright sunlight for patients with migraine, tension-type headaches, and those with light sensitivity. Some tints have been successful in migraine. Good et al (29) found that FL-41 tint, a rose-colored tint, reduced migraine frequency in children by more than one half. Sub-jects reported a decrease in photophobia and glare in between attacks but no change in the light sensitivity associated with the migraine attack. FL-41 tint filters 80% of short wave-length 50 or 60 Hz flicker that is seen with fluorescent lights. As flicker stimuli can be particularly noxious to patients with migraine (125), the authors reasoned that flicker reduction contributed to the reduction in headaches. We studied FL-41-tinted lenses and found that they in-creased the threshold to discomfort in all subjects (controls, migraineurs, and patients with blepharospasm), but they did not differ from gray-tinted lenses in reducing light sensitiv-ity (44). To test whether patients preferred FL-41-tinted over gray-tinted spectacles, we performed a double cross-over study of subjects with blepharospasm using gray and FL-41 tint. Patients preferred FL-41 tint over gray specta-cles, and they felt that FL-41 significantly reduced their symptoms (70). We also tested the blink reflexes of patients who wore FL-41 tint or placebo pink lenses while reading under a standardized light source. We found that in bleph-arospasm patients, FL-41 tint greatly reduced the number of blinks and intensity of blinks (70). Studies using fMRI suggest that there may be different physiologic responses to spectrally specific tints compared to neutral density filtering (which attenuates all wavelengths 76 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. equally). Huang et al (126) found that precision ophthalmic tints normalized cortical activation on fMRI, whereas gray lenses did not, in patients with migraine. Why would red-or pink-tinted lenses show this effect? Red tints tend to block blue wavelengths, which more likely may induce photophobia (103). Treatment of Dry Eyes Aggressive treatment of dry eyes may be helpful. Blepharo-spasm- associated dry eyes are commonly treated with drops and ointments and possible punctual plugs (127). Anti-inflammatory drops have been tested in reducing light sensitivity after cataract surgery and have not been found to be helpful (128). Xylocaine has been used after cataract surgery without decrease in light sensitivity (129). Dilating Drops Cycloplegics can be used to give some relief in patients with ocular inflammation. This is likely due to reduction in ciliary muscle spasm since papillary dilation actually increases light entering the eye. Systemic Medications Sedatives (e.g., barbiturates, benzodiazepines) that reduce "tri-geminal irritability" have been reported to be helpful and allow for prolonged sleep and closed eyes (130). However caution should be exercised with these kinds of medication. Treatment of migraine-associated photophobia with migraine preventive medications including beta-blockers, calcium chan-nel blockers, and anti-convulsants is reasonable (96). Acute migraine should be treated with migraine-specific medications that have been shown to reduce photophobia associated with an acute attack (96). To the extent that other photophobic disorders are due to dysfunctional excitation/inhibition bal-ance, migraine preventives, especially of the antiepileptic class, might be considered. Systemic medications that have been anecdotally reported to reduce the pain associated with pho-tophobia include gabapentin and melatonin. FIG. 2. An approach to the patient with photophobia. Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 77 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Given the alleviation of photophobia with antidepressants in patients with comorbid depression (49,50), such treatment appears helpful. If anxiety and panic disorder are present, anxiolytics may be beneficial. While the mainstay treatment of blepharospasm is botulinum toxin (127), clinicians have tried tricyclic antidepressants, baclofen, benzodiazepine, orphenadrine, carbidopa/levodopa, and fluoxetine (131). If corneal neuropathy is suspected, Rosenthal suggests topical lacosamide or systemic anticonvulsants, such as gabapentin, pregabalin, or carbamazepine (15). Procedures Various techniques have been described in treating photo-phobia in difficult clinical settings. Injections to the supraorbital nerve have been reported to cause a reduction in light sensitivity (130). Alcohol (40%-60%) injected into the orbit (1.5 mL) has been reported to be helpful in cases of ocular inflammation to reduce photophobia and did not influence visual acuity (132). In one series, the majority of individuals with whiplash-induced cervicalgia and photo-phobia found relief with trigger point injections (133). Bot-ulinum toxin injection was found to reduce photophobia associated with posttraumatic headache (134) and is used to treat chronic migraine (135). Sympathetic blockade was first reported by Magitot (136). Fine and Digre (9) showed that superior cervical blockade by lidocaine did improve light sensitivity in some patients with photophobia. This treatment may be best in patients who have had known injury to the anterior seg-ment and who experience continued photo-oculodynia despite complete resolution of the injury. ACKNOWLEDGMENTS The authors thank Susan Schulman for her expert editorial assistance. They also thank Dr. Bradley Katz for his suggestions. REFERENCES 1. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(suppl 7):1-96. 2. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. 2004;24:1-160. 3. Trobe JD. Photophobia in anterior visual pathway disease. J Neuroophthalmol. 2002;22:1-2. 4. Webster's New World Dictionary. Cleveland, OH: The World Publishing Company, 1968. 5. Lebensohn J. The nature of photophobia. Arch Ophthalmol. 1934;12:380-383. 6. Cummings JL, Gittinger JW Jr. Central dazzle. A thalamic syndrome? Arch Neurol. 1981;38:372-374. 7. Loewenfeld I. The "dazzling" syndrome. In: The Pupil: Anatomy, Physiology, and Clinical Applications. Detroit, MI: Wayne State University Press, 1993:1200. 8. Ohba N, Ohba A. Nyctalopia and hemeralopia: the current usage trend in the literature. Br J Ophthalmol. 2006;90:1548-1549. 9. Fine PG, Digre KB. A controlled trial of regional sympatholysis in the treatment of photo-oculodynia syndrome. J Neuroophthalmol. 1995;15:90-94. 10. Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(pt 8):1703-1709. 11. Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004;63:848-852. 12. Buchanan T, Digre K, Warner JE, Katz BJ. A review of the symptom of photophobia in an eye clinic. Neurology. 2009;72:A184. 13. Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2011;152:900-909 e901. 14. Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, Garcia-Sanchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173-181. 15. Karmel M. Addressing the pain of corneal neuropathy [online]. Available at: http://www.aao.org/publications/ eyenet/201007/upload/29_CUcornea_FF_copy.pdf. Accessed November 18, 2011. 16. Prokofyeva E, Troeger E, Wilke R, Zrenner E. Early visual symptom patterns in inherited retinal dystrophies. Ophthalmologica. 2011;226:151-156. 17. Zervas JP, Smith JL. Neuro-ophthalmic presentation of cone dysfunction syndromes in the adult. J Clin Neuroophthalmol. 1987;7:202-218. 18. Simunovic MP, Moore AT. The cone dystrophies. Eye (Lond). 1998;12(pt 3b):553-565. 19. Russell-Eggitt IM, Clayton PT, Coffey R, Kriss A, Taylor DS, Taylor JF. Alstrom syndrome. Report of 22 cases and literature review. Ophthalmology. 1998;105:1274-1280. 20. Lamonte M, Silberstein SD, Marcelis JF. Headache associated with aseptic meningitis. Headache. 1995;35:520-526. 21. Welty TE, Horner TG. Pathophysiology and treatment of subarachnoid hemorrhage. Clin Pharm. 1990;9:35-39. 22. Kawasaki A, Purvin VA. Photophobia as the presenting visual symptom of chiasmal compression. J Neuroophthalmol. 2002;22:3-8. 23. Fricke B, Andres KH, Von During M. Nerve fibers innervating the cranial and spinal meninges: morphology of nerve fiber terminals and their structural integration. Microsc Res Tech. 2001;53:96-105. 24. Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia. 2009;29:953-959. 25. Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, Harrison W. A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology. 2003;61:375-382. 26. Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13:321-324. 27. Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733-741. 28. Vincent AJ, Spierings EL, Messinger HB. A controlled study of visual symptoms and eye strain factors in chronic headache. Headache. 1989;29:523-527. 29. Good PA, Taylor RH, Mortimer MJ. The use of tinted glasses in childhood migraine. Headache. 1991;31:533-536. 30. Chronicle EP, Mulleners WM. Visual system dysfunction in migraine: a review of clinical and psychophysical findings. Cephalalgia. 1996;16:525-535; discussion 523. 31. Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465-469. 78 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 32. Irimia P, Cittadini E, Paemeleire K, Cohen AS, Goadsby PJ. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia. 2008;28:626-630. 33. Cittadini E, Goadsby PJ. Hemicrania continua: a clinical study of 39 patients with diagnostic implications. Brain. 2010;133:1973-1986. 34. Waddell PA, Gronwall DM. Sensitivity to light and sound following minor head injury. Acta Neurol Scand. 1984;69:270-276. 35. Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, Stepan ChA, Traubner P, Twijnstra A, Vecsei L, von Wild K. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9:207-219. 36. Bohnen N, Twijnstra A, Wijnen G, Jolles J. Tolerance for light and sound of patients with persistent post-concussional symptoms 6 months after mild head injury. J Neurol. 1991;238:443-446. 37. Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300:711-719. 38. Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453-463. 39. Ruff RL, Ruff SS, Wang XF. Headaches among Operation Iraqi Freedom/Operation Enduring Freedom veterans with mild traumatic brain injury associated with exposures to explosions. J Rehabil Res Dev. 2008;45:941-952. 40. Neely ET, Midgette LA, Scher AI. Clinical review and epidemiology of headache disorders in US service members: with emphasis on post-traumatic headache. Headache. 2009;49:1089-1096. 41. Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985;108(pt 3):593-608. 42. Anderson RL, Patel BC, Holds JB, Jordan DR. Blepharospasm: past, present, and future. Ophthal Plast Reconstr Surg. 1998;14:305-317. 43. Judd R, Digre KB, Warner JE, Schulman S, Katz BJ. Shedding light on blepharospasm: a patient-researcher partnership approach to assessment of photophobia and impact on activities of daily living. Neuroophthalmology. 2007;31:49-54. 44. Adams WH, Digre KB, Patel BC, Anderson RL, Warner JE, Katz BJ. The evaluation of light sensitivity in benign essential blepharospasm. Am J Ophthalmol. 2006;142:82-87. 45. Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord. 2009;15:59-61. 46. Hills W, Warner JE, Katz BJ, Chelune G, Foster N, Steffens J, Thulin P, Chin S, Digre K. Neuro-Ophthalmic Findings That May Reliably Differentiate Progressive Supranuclear Palsy and Parkinson's Disease. Chicago, IL: American Academy of Neurology, 2008. 47. Kellner M, Wiedemann K, Zihl J. Illumination perception in photophobic patients suffering from panic disorder with agoraphobia. Acta Psychiatr Scand. 1997;96:72-74. 48. Shirani A, St Louis EK. Illuminating rationale and uses for light therapy. J Clin Sleep Med. 2009;5:155-163. 49. Gerbaldo H. Depression and photophobic behavior. Am J Psychiatry. 1988;145:1479-1480. 50. Gerbaldo H, Thaker G. Photophilic and photophobic behaviour in patients with schizophrenia and depression. Can J Psychiatry. 1991;36:677-679. 51. Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119:202-210. 52. Bossini L, Fagiolini A, Valdagno M, Padula L, Hofkens T, Castrogiovanni P. Photosensitivity in panic disorder. Depress Anxiety. 2009;26:E34-E36. 53. Bengtzen R, Woodward M, Lynn MJ, Newman NJ, Biousse V. The "sunglasses sign" predicts nonorganic visual loss in neuro-ophthalmologic practice. Neurology. 2008;70:218-221. 54. Schutz JS, Mavrakanas NA. The value of the ophthalmological independent medical examination: analysis of 344 cases. Br J Ophthalmol. 2009;93:1371-1375. 55. Weller M, Wiedemann P. Hysterical symptoms in ophthalmology. Doc Ophthalmol. 1989;73:1-33. 56. Burton H. Somatic sensation from the eye. In: Hart WM, ed. Adler's Physiology of the Eye. St Louis, MO: Mosby Year Book, 1992:71-100. 57. Last R. Eugene Wolff's Anatomy of the Eye and Orbit, 5th edition. Philadelphia, PA: W.B. Saunders, 1961. 58. Edvinsson L, Uddman R. Neurobiology in primary headaches. Brain Res Brain Res Rev. 2005;48:438-456. 59. Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161:327-341. 60. Goadsby PJ. Pathophysiology of migraine. Neurol Clin. 2009;27:335-360. 61. Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12:570-584. 62. Marfurt CF. Sympathetic innervation of the rat cornea as demonstrated by the retrograde and anterograde transport of horseradish peroxidase-wheat germ agglutinin. J Comp Neurol. 1988;268:147-160. 63. Davis L, Pollock L. The rôle of the sympathetic nervous system in the production of pain in the head. Arch Neurol Psychiatry. 1932;27:282-293. 64. McCann JD, Gauthier M, Morschbacher R, Goldberg RA, Anderson RL, Fine PG, Digre KB. A novel mechanism for benign essential blepharospasm. Ophthal Plast Reconstr Surg. 1999;15:384-389. 65. Esteban A. A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin. 1999;29:7-38. 66. Yates SK, Brown WF. Light-stimulus-evoked blink reflex: methods, normal values, relation to other blink reflexes, and observations in multiple sclerosis. Neurology. 1981;31:272-281. 67. Bologna M, Agostino R, Gregori B, Belvisi D, Ottaviani D, Colosimo C, Fabbrini G, Berardelli A. Voluntary, spontaneous and reflex blinking in patients with clinically probable progressive supranuclear palsy. Brain. 2009;132:502-510. 68. Pearce JM. Observations on the blink reflex. Eur Neurol. 2008;59:221-223. 69. Aramideh M, Ongerboer de Visser BW. Brainstem reflexes: electrodiagnostic techniques, physiology, normative data, and clinical applications. Muscle Nerve. 2002;26:14-30. 70. Blackburn MK, Lamb RD, Digre KB, Smith AG, Warner JE, McClane RW, Nandedkar SD, Langeberg WJ, Holubkov R, Katz BJ. FL-41 tint improves blink frequency, light sensitivity, and functional limitations in patients with benign essential blepharospasm. Ophthalmology. 2009;116:997-1001. 71. Siegwart K. Zur Frage nach dem Vorkommen and dem Wesen des Blednungsschmerzes. Schweizmed Wchschr. 1920;50:1165. 72. Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gosley JJ, Brainard GS, Gregory-Evans K, Rizzo J, Czeisler CA, Foster RG, Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122-2128. 73. Noseda R, Burstein R. Advances in understanding the mechanisms of migraine-type photophobia. Curr Opin Neurol. 2011;24:197-202. 74. Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: an alternate visual pathway. Case report. J Neurosurg. 2006;105:765-768. 75. Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Lido HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 79 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Berson DM, Lucas RJ, Yau KW, Haltar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102-105. 76. Wilhelm H. The pupil. Curr Opin Neurol. 2008;21:36-42. 77. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009;116:1564-1573. 78. Kawasaki A, Kardon RH. Intrinsically photosensitive retinal ganglion cells. J Neuroophthalmol. 2007;27:195-204. 79. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070-1073. 80. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065-1070. 81. Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547-1581. 82. Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92-95. 83. Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572-580. 84. Xue T, Do MT, Riccio A, Jiang Z, Hsieh J, Wang HC, Merbs SL, Welsbie DS, Yoshioka T, Weissgerber P, STolz S, Flockerzi V, Friechel M, Simon MI, Clapham DE, Yau KW. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67-73. 85. Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160: 858-864. 86. Noseda R, Constandil L, Bourgeais L, Chalus M, Villanueva L. Changes of meningeal excitability mediated by corticotrigeminal networks: a link for the endogenous modulation of migraine pain. J Neurosci. 2010;30:14420-14429. 87. Hirata A, Aguilar J, Castro-Alamancos MA. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci. 2006;26:4426-4436. 88. Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297-3308. 89. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679-685. 90. Nicolelis MA, Fanselow EE. Thalamocortical [correction of thalamcortical] optimization of tactile processing according to behavioral state. Nat Neurosci. 2002;5:517-523. 91. Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52:7852-7858. 92. Custer PL, Reistad CE. Enucleation of blind, painful eyes. Ophthal Plast Reconstr Surg. 2000;16:326-329. 93. Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104-1110. 94. Ho TW, Ferrari MD, Dodick DW, Galet V, Kost J, Fan X, Leibensperger H, Froman S, Assaid C, Lines C, Koppen H, Winner PK. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115-2123. 95. Russo AF, Kuburas A, Kaiser EA, Raddant AC, Recober A. A potential preclinical migraine model: CGRP-sensitized mice. Mol Cell Pharmacol. 2009;1:264-270. 96. Sprenger T, Goadsby PJ. Migraine pathogenesis and state of pharmacological treatment options. BMC Med. 2009;7:71. 97. Recober A, Kaiser EA, Kuburas A, Russo AF. Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 2010;58:156-165. 98. Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798-8804. 99. Vanagaite Vingen J, Stovner LJ. Photophobia and phonophobia in tension-type and cervicogenic headache. Cephalalgia. 1998;18:313-318. 100. Wirtschafter JD, Bourassa CM, Dow RS. The quantitative study of photophobia. Trans Am Neurol Assoc. 1963;88:291-293. 101. Marks L. Brightness and equivalent intensity of intrinsic light. Vision Res. 1973;13:371-382. 102. Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23:307-380. 103. Main A, Vlachonikolis I, Dowson A. The wavelength of light causing photophobia in migraine and tension-type headache between attacks. Headache. 2000;40:194-199. 104. Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Jassen S, Gerna E, Rollag MD. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J Biol Rhythms. 2008;23:379-386. 105. Kowacs PA, Piovesan EJ, Werneck LC, Tatsui CE, Lange MC, Ribas LC, da Silva HP. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184-188. 106. Moulton EA, Becerra L, Borsook D. An fMRI case report of photophobia: activation of the trigeminal nociceptive pathway. Pain. 2009;145:358-363. 107. Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111-124. 108. Malecaze FJ, Boulanouar KA, Demonet JF, Guell JL, Imbert MA. Abnormal activation in the visual cortex after corneal refractive surgery for myopia: demonstration by functional magnetic resonance imaging. Ophthalmology. 2001;108:2213-2218. 109. Emoto H, Suzuki Y, Wakakura M, Horie C, Kiyosawa M, Mochizuki M, Kawasaki K, Oda K, Ishiwata K, Ishii K. Photophobia in essential blepharospasm-a positron emission tomographic study. Mov Disord. 2010;25:433-439. 110. Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427-1439. 111. Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9:344-352. 112. Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischi B, Kwang KK, Cotren FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687-4692. 113. Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Geraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology. 2011;76:213-218. 114. Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81:978-984. 115. Brennan KC. Turn down the lights! An irritable occipital cortex in migraine without aura. Neurology. 2011;76:206-207. 116. Martin H, Del Rio MS, de Silanes CL, Alvarez-Linera J, Hernandez JA, Pareja JA. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: pathophysiological implications. Headache. 2011;51:1520-1528. 80 Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 117. Llop S, Frandsen F, Katz BJ, Warner JE, Digre K. Depression and anxiety in migraine patients with and without chronic photophobia. Headache. 2010;50:S45. 118. Bruce BB, Newman NJ. Functional visual loss. Neurol Clin. 2010;28:789-802. 119. Howard RJ, Valori RM. Hospital patients who wear tinted spectacles-physical sign of psychoneurosis: a controlled study. J R Soc Med. 1989;82:606-608. 120. Marshall JD, Paisey RB, Carey C, Macdermott S. Alstrom syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, eds. GeneReviews [Internet]. Seattle, WA: University of Washington, 1993-2003; [updated June 08, 2010]. 121. Rajak SN, Currie AD, Dubois VJ, Morris M, Vickers S. Tinted contact lenses as an alternative management for photophobia in stationary cone dystrophies in children. J AAPOS. 2006;10:336-339. 122. Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88:291-297. 123. Young RS, Krefman RA, Fishman GA. Visual improvements with red-tinted glasses in a patient with cone dystrophy. Arch Ophthalmol. 1982;100:268-271. 124. Park WL, Sunness JS. Red contact lenses for alleviation of photophobia in patients with cone disorders. Am J Ophthalmol. 2004;137:774-775. 125. Karanovic O, Thabet M, Wilson HR, Wilkinson F. Detection and discrimination of flicker contrast in migraine. Cephalalgia. 2011;31:723-736. 126. Huang J, Zong X, Wilkins A, Jenkins B, Bozoki A, Cao Y. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia. 2011;31:925-936. 127. Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology. 2008;71:1275-1282. 128. McDonald MB, Wyse TB, Borodkin MJ, Ocmand A, Shoelson B, Thompson H. Comparison of the effectiveness of 4 anti-inflammatory drops in relieving photophobia after pupil dilation. J Cataract Refract Surg. 1999;25:405-410. 129. Brierley L. Effect of lidocaine on photophobia. J Cataract Refract Surg. 1999;25:1177. 130. Lebensohn JE. Photophobia: mechanism and implications. Am J Ophthalmol. 1951;34:1294-1300. 131. Spencer BR Jr, Digre KB. Treatments for neuro-ophthalmologic conditions. Neurol Clin. 2010;28:1005-1035. 132. Hoyt W. Walsh and Hoyt's Neuro-Ophthalmology, 3rd edition. Philadelphia, PA: Lippincott Williams and Wilkins, 1969. 133. Freeman MD, Nystrom A, Centeno C. Chronic whiplash and central sensitization; an evaluation of the role of a myofascial trigger points in pain modulation. J Brachial Plex Peripher Nerve Inj. 2009;4:2. 134. Shoari M, Katz BJ. Treatment of the photo-oculodynia syndrome with botulinum toxin A. Neuro-Ophthalmology. 2007;31:105-110. 135. Diener HC, Dodick DW, Aurora SK, Turkell CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804-814. 136. Magitot A. Photophobie. Ann D'ocul. 1937;174:917. 137. Tugal-Tutkun I, Araz B, Taskapili M, Akora YA, Yalniz-Alkkaya Z, Berkeu N, Emie S, Gezer A. Bilateral acute depigmentation of the iris: report of 26 new cases and four-year follow-up of two patients. Ophthalmology. 2009;116:1552-1557, 1557 e1551. 138. Soeters MR, van Zeijl CJ, Boelen A, Kloos R, Saeed P, Vriesendrop TM, Mourits MP. Optimal management of Graves orbitopathy: a multidisciplinary approach. Neth J Med. 2011;69:302-308. 139. Enkvetchakul O, Thanathanee O, Rangsin R, Lekhanont K, Suwan-Apichon O. A randomized controlled trial of intralesional bevacizumab injection on primary pterygium: preliminary results. Cornea. 2011;30: 1213-1218. 140. van der Veen RL, Fuijkschot J, Willemsen MA, Cruysberg JR, Berendschot TT, Theelen T. Patients with Sjogren-Larsson syndrome lack macular pigment. Ophthalmology. 2010;117:966-971. 141. Kaur H, Uy C, Kelly J, Moses AM. Orbital inflammatory disease in a patient treated with zoledronate. Endocr Pract. 2011;17:e101-e103. 142. Alshami M, Bawazir MA, Atwan AA. Photoletter to the editor: a new variant of ichthyosis follicularis with alopecia and photophobia (IFAP) syndrome with coexisting psoriasiform lesions and palmoplantar keratoderma. IFAP-PPK syndrome? J Dermatol Case Rep. 2011;5:14-16. 143. Megarbane H, Megarbane A. Ichthyosis follicularis, alopecia, and photophobia (IFAP) syndrome. Orphanet J Rare Dis. 2011;6:29. 144. Carey J. Trisomy 18 and trisomy 13 syndromes. In: Cassidy S, Allanson J, eds. Management of Genetic Syndromes, 3rd edition. Hoboken, NJ: Wiley-Blackwell, 2010:807-825. 145. Yu HH, Yang TM, Shan YS, Lin PW. Zinc deficiency in patients undergoing pancreatoduodenectomy for periampullary tumors is associated with pancreatic exocrine insufficiency. World J Surg. 2011;35:2110-2117. Digre and Brennan: J Neuro-Ophthalmol 2012; 32: 68-81 81 State-of-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |