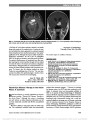
| OCR Text |
Show Primary Angiitis of the Central Nervous System Presenting as Unilateral Optic Neuritis Neal M. Rao, MD, Pradeep S. Prasad, MD, Charles C. Flippen II, MD, Aaron S. Wagner, MD, Catherine M. Yim, MD, Noriko Salamon, MD, Harry V. Vinters, MD Abstract: A middle-aged woman who experienced recurrent episodes of unilateral vision loss and eye pain. On pre-sentation, magnetic resonance imaging (MRI) demonstrated left optic nerve enhancement with patchy hyperintensities in the white matter of both frontal lobes and ill-defined enhancement in a lenticulostriate distribution. Ophthalmo-logic examination revealed left optic disc edema with a macular scar consistent with neuroretinitis. Her sub-sequent clinical course was notable for 2 episodes of painful vision loss, without associated neurologic symp-toms, which resolved with intravenous and oral steroids. More than 1 year after her initial presentation, the patient developed right facial weakness and slurred speech, and shortly thereafter suffered a fatal intracerebral hemorrhage. Histopathology on autopsy confirmed a diagnosis of primary angiitis of the central nervous system (PACNS). This is an unusual case of PACNS presenting with recurrent unilateral optic neuritis. The vascular enhancement pattern on MRI suggesting inflamed cerebral blood vessels is a rarely described pattern, which likely reflects intracerebral exten-sion of the ocular pathology. The combination of neuro-retinitis and perivascular MRI enhancement pattern may represent a subtype of PACNS. Journal of Neuro-Ophthalmology 2014;34:380-385 doi: 10.1097/WNO.0000000000000147 © 2014 by North American Neuro-Ophthalmology Society Primary angiitis of the central nervous system (PACNS) is a rare disorder. A constellation of symptoms may be seen, including headache and encephalopathy, as well as focal neurological deficits such as stroke, transient ischemic attack, seizure, and myelopathy (1,2). Magnetic resonance imaging (MRI) frequently is abnormal; yet, the abnormal-ities are heterogeneous, involving virtually all parts of the central nervous system (2-5). We describe a case of PACNS with several unique features, including inflammation along the lenticulostriate and retinal arteries. CASE REPORT Three weeks before presentation, a 54-year-old woman, with medical history significant only for irritable bowel syndrome, noted 2 small areas of visual obscuration in the lower left quadrant of her visual field in the left eye. Over the next 2 weeks there was a slow spread of this defect to encompass the central left visual field. One week before presentation, she reported dull pain with movement of the left eye, followed by a mild bitemporal headache. She was of Swedish origin and without a family history of neurological disease. On examination, her vital signs and general and neurological examination were unremarkable. Visual acuity was 20/25, right eye, and counting fingers, left eye. Extraocular movements were intact. The right fundus showed mild retinal vascular tortuosity, whereas the left eye revealed an edematous optic disc with associated peripapillary hemorrhages and a macular star (Fig. 1). Brain MRI showed periventricular white matter hyperintensities and, after intravenous contrast, a lenticulostriate perivascu-lar enhancement pattern and left optic nerve enhancement (Fig. 2). Complete blood count, rapid plasma regain, fluo-rescent treponemal antibody-absorption test, and HIV 1 and 2 tests were negative. Inflammatory markers were sim-ilarly not significant with C-reactive protein ,0.5 mg/dL (normal ,0.8 mg/dL) and erythrocyte sedimentation rate of 1 mm/hr. Rheumatologic workup with antinuclear anti-body, SSA/SSB, SCL 70, antineutrophil cytoplasmic anti-body and antidouble stranded DNA were negative. Workup for disorders of coagulation including protein S and C, anti-phospholipid antibody, B2 glycoprotein, antithrombin III, Departments of Neurology (NMR, CF, HVV), and Ophthalmology (PP, ASW), David Geffen School of Medicine at UCLA, Los Angeles, California; Department of Pathology (PP, ASW), Orlando Regional Medical Center, Orlando, Florida; Departments of Pathology and Laboratory Medicine (HVV), and Radiological Sciences (CY, NS), David Geffen School of Medicine at UCLA, Los Angeles, California. The authors report no conflicts of interest. Address correspondence to Neal M. Rao, MD, UCLA Stroke Center, 710 Westwood Plaza, Reed Building, Office 4-126, Los Angeles, CA 90095; E-mail: nealrao@mednet.ucla.edu 380 Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 1. There is venous engorgement and tortuosity in the right eye and optic disc edema with a macular star (arrow) in the left eye. FIG. 2. Initial magnetic resonance imaging (MRI). A. Axial fluid-attenuated inversion recovery image shows periventricular white matter hyperintensities. B. Postcontrast T1 axial MRI demonstrates perivascular enhancement in a lenticulostriate distribution (arrow). Enhancement of the optic disc (arrow) (C) and orbital optic nerve (arrow) (D) is present on axial and coronal images, respectively. Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 381 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. factor V Leiden, prothrombin mutation, and homocysteine were all negative or normal. Angiotensin-converting enzyme (ACE) was 49 U/L (normal: 10-66 U/L). Chest radiography was unremarkable. Lumbar puncture (LP) showed a red blood cell count of 1/mm3 and a white blood cell count of 11/mm3 (91% lymphocytes and 9% monocytes), glucose of 71 mg/dL (normal: 63-73 mg/dL), and protein of 46 mg/dL (normal: 15-45 mg/dL). Gram stain, fungal, bacterial, viral, and acid-fast bacilli cultures were negative. Coccidioides and Cryptococcus titers were negative. Because of worsen-ing visual acuity, the patient was given a 3-day course of 1 g of intravenous solumedrol. A second high volume LP was performed demonstrating 1 red cell per cubic milli-meter and 18 white cells per cubic millimeter with 95% lymphocytes and 5% monocytes. CSF glucose was 88 mg/ dL, and protein was 42 mg/dL. Cytology was negative. IgG synthesis rate was normal, myelin basic protein and neuromyelitis optica IgG were negative. No oligoclonal bands were present. CSF ACE and VDRL were negative. Polymerase chain reaction evaluation of the CSF for Lyme, HSV, VZV, EBV, MV, and HTLV I/II were negative as well. After steroid administration, the patient's vision improved. One month after her initial presentation, MRI showed periventricular white matter hyperintensities, and contrast-enhanced MR angiography did not show any evi-dence of vasculopathy (Fig. 3). One year later, the patient noted decreased visual acuity in her right eye with bilateral discomfort with eye movement. Visual acuity was 20/50, right eye and 20/30, FIG. 3. After corticosteroid treatment, magnetic resonance imaging (MRI) reveals near complete resolution of periventricular white matter changes on T2 axial MRI (A) and decreased perivascular enhancement on postcontrast T1 MRI (B). MRA (C, D) shows normal cerebral vasculature. 382 Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. left eye. No relative afferent pupillary defect was noted, and her neurological examination was normal. On fundus examination, there was optic disc edema and a branch retinal artery occlusion in the right eye. Repeat laboratory studies were without significant abnormalities. Solumedrol 1 g IV was administered daily for 3 days with subsequent resolution of symptoms. One month later, she again presented with subjective worsening of visual acuity without objective change. She was started on daily oral prednisone, which was continued for 2 months. Serial ophthalmologic FIG. 4. Approximately 14 months after initial presentation, magnetic resonance imaging (MRI) shows increased white matter hyperintensities on axial fluid-attenuated inversion recovery image imaging (A), perivascular contrast enhancement on T1 axial image (B), and petechial hemorrhages in the right frontal and parietal subcortical white matter on gradient echo scan (C). FIG. 5. Leptomeningeal blood vessels stained with hematoxylin and eosin. A. The vessel wall is heavily infiltrated by inflammatory cells (arrows) with obliteration of the lumen. An adjacent artery (lower left) is unaffected. B. Tangential section of artery with chronic transmural and adventitial inflammation. Arrows indicate a luminal thrombus. C. Magnified view to show vascular inflammation with mural thrombus. Arrow indicates a nonocclusive platelet-fibrin thrombus at the luminal edge. Scale bars represent 100 mm. Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 383 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. examinations were performed, and steroids were tapered as visual acuity returned to baseline. Four months after discharge, the patient reported pro-gressive tingling in her right hand and both feet and experienced a 2-minute episode of slurred speech with right lower facial droop. Brain MRI showed worsening white matter disease (Fig. 4). Before readmission to our hospital, the patient suffered a generalized tonic-clonic sei-zure and was taken to another hospital. She was found to have a large right frontal hemorrhage with midline shift and expired shortly thereafter from herniation with brainstem compression. A limited autopsy of the brain was performed. Gross dissection revealed a 9-cm hemorrhagic, liquefied, blood-filled cavity involving the right frontal and parietal lobes. The venous sinuses and proximal cerebral vessels were patent without evidence of thrombosis. Cultures for tuberculosis, fungi and bacteria were negative. Microscopy did not reveal any evidence of lymphoma or other neoplasm. Histopathology of the brain demonstrated extensive infiltration of the leptomeningeal and cerebral blood vessel walls with inflammatory cells, including acute (polymorphonuclear leukocytes) and chronic (lympho-cytes and monocytes) elements. There was evidence of chronic transmural and adventitial inflammation, intimal thickening, and partial or complete thrombosis of multiple vessels (Fig. 5). There was extensive, multifocal loss of elastic fibers in multiple vessels throughout the brain (Fig. 6). There also were some areas with fragmentation of the elastic fibers. The pathology was most consistent with PACNS. DISCUSSION Our patient with PACNS presented with visual loss due to neuroretinitis. Initially, her only complaints were a mild headache and decreased vision in her left eye associated with pain on eye movement. She did not display any of the typical findings of PACNS, such as encephalopathy or other focal neurologic findings. Although the initial symptoms and neurological examination raised suspicion for demye-linating optic neuritis, there were some atypical findings. The ophthalmologic examination showed disc hemorrhages and a macular star, which argued strongly against a demy-elinating etiology (6,7). The MRI demonstrated contrast enhancement in a perivascular pattern, raising concern for-an infectious, neoplastic, or vasculitic process. In the absence of infectious markers in this otherwise asymptom-atic patient, PACNS was the most likely diagnosis, which ultimately was confirmed at autopsy. PACNS is a heterogeneous disorder with clinical subsets involving small or medium-sized vessels. The associated MRI findings are similarly diverse with patients showing white matter involvement with areas of contrast enhancement, and/or leptomeningeal involvement, with or without angiographic findings (2-5). Patterns of clinical presentation and radiographic findings may signify distinct subtypes of PACNS, some of which may be associated FIG. 6. Sections stained for elastic (van Gieson). A. Two arteries (arrows) show extensive loss of elastic fibers with intimal thickening. B. Longitudinal section reveals thickening and chronic scarring of vessel wall with loss of elastic fibers (arrow-heads) and extensive transmural inflammation (arrows). Scale bars represent 100 mm. 384 Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. with ocular pathology. There are reports of optic disc edema in encephalopathic PACNS patients having increased intra-cranial pressure (8-11), occasionally with large vessel abnor-malities detected on cerebral angiography (8). Hassan et al (12) described a patient with a linear pattern of cerebral contrast enhancement on MRI and bilateral optic disc edema with normal intracranial pressure. The optic disc edema was thought to be due to vasculitic inflammation of the optic nerves. In our patient, vascular inflammation could be directly observed in the small vessels of the retina including retinal hemorrhages and a branch retinal artery occlusion. This constellation of features, primarily involving the small vessels, indicates a possible subset of PACNS with small vessel predominance. Our report highlights the importance of ophthalmologic examination of patients suspected of having cerebral vasculitis. In patients with perivascular contrast enhance-ment on MRI and normal cerebrovascular imaging, a careful retinal examination may be the only way to directly observe small vessel involvement. In such cases, fluorescein angiog-raphy has been suggested as a potential diagnostic tool (13). The presence of retinal vasculitis or retinal vascular occlu-sion along with a linear enhancement pattern seen on MRI should increase the suspicion for PACNS as a possible unifying diagnosis. REFERENCES 1. Molloy ES, Hajj-Ali RA. Primary angiitis of the central nervous system. Curr Treat Options Neurol. 2007;9:169-175. 2. Birnbaum J, Hellmann DB. Primary angiitis of the central nervous system. Arch Neurol. 2009;66:704-709. 3. Küker W. Imaging of cerebral vasculitis. Int J Stroke. 2007;2:184-190. 4. Hajj-Ali RA, Calabrese LH. Central nervous system vasculitis. Curr Opin Rheumatol. 2009;21:10-18. 5. Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH. Primary angiitis of the CNS. Lancet Neurol. 2011;10:561-572. 6. Brazis PW, Lee AG. Optic disk edema with a macular star. Mayo Clin Proc. 1996;71:1162-1166. 7. Parmley VC, Schiffman JS, Maitland CG, Miller NR, Dreyer RF, Hoyt WF. Does neuroretinitis rule out multiple sclerosis? Arch Neurol. 1987;44:1045-1048. 8. Burger PC, Burch JG, Vogel FS. Granulomatous angiitis. An unusual etiology of stroke. Stroke. 1977;8:29-35. 9. Hughes JT, Brownell B. Granulomatous giant-celled angiitis of the central nervous system. Neurology. 1966;16:293-298. 10. Rawlinson DG, Braun CW. Granulomatous angiitis of the nervous system first seen as relapsing myelopathy. Arch Neurol. 1981;38:129-131. 11. Kolodny EH, Rebeiz JJ, Caviness VS Jr, Richardson EP Jr. Granulomatous angiitis of the central nervous system. Arch Neurol. 1968;19:510-524. 12. Hassan AS, Trobe JD, McKeever PE, Gebarski SS. Linear magnetic resonance enhancement and optic neuropathy in primary angiitis of the central nervous system. J Neuroophthalmol. 2003;23:127-131. 13. Scolding NJ, Jayne DRW, Zajicek JP, Meyer PAR, Wraight EP, Lockwood CM. Cerebral vasculitis-recognition, diagnosis, and management. QJM. 1997;90:61-73. Rao et al: J Neuro-Ophthalmol 2014; 34: 380-385 385 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |