| OCR Text |
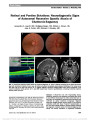
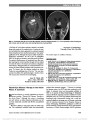
Show IgG4-Related Disease: A Neuro-Ophthalmological Perspective Satoshi Kashii, MD, PhD Abstract: Immunoglobulin G4-related disease (IgG4-RD) is a multifocal inflammatory disorder that causes tumefac-tive lesions with a dense lymphoplasmacytic infiltrate rich in IgG4 plasma cells and storiform-pattern fibrosis. The clinical symptoms are relatively mild, and the condition is usually recognized by organ swelling and damage. When referring to the ophthalmic manifestations of IgG4-RD, the term IgG4-related ophthalmic disease (IgG4-ROD) is used. IgG4-ROD is characterized by bilateral lacrimal gland enlargement accompanied by 3 distinctive features: infraorbital nerve enlargement, extraocular myo-sitis, and compressive optic neuropathy. IgG4 implies an underlying systemic disease process requiring evaluation to detect other systemic involvement. This includes hypo-physitis and hypertrophic pachymeningitis, entities of neuro-ophthalmic interest. IgG4-ROD usually responds favorably to systemic corticosteroids but may be compli-cated by relapse during steroid taper. Rituximab has been shown to be effective for controlling steroid-refractory IgG4-RD. In contrast to IgG4-RD, an increasing number of cases of extranodal marginal B-cell lymphoma (MALT type) associated with IgG4-ROD have been described. IgG4 may be a risk factor for later emergence of low-grade B-cell lymphoma. Journal of Neuro-Ophthalmology 2014;34:400-407 doi: 10.1097/WNO.0000000000000193 © 2014 by North American Neuro-Ophthalmology Society Acompanion article by Yamamoto et al (1) presents an historical and general overview of IgG4-related disease (IgG4-RD). Neuro-ophthalmic manifestations of this dis-order are the subject of this review. IgG4-RELATED OPHTHALMIC DISEASE In 2011, the organizing committee of the International Symposium on IgG4-RD adopted the term IgG4-RD to describe a multifocal inflammatory disorder that causes tumefactive lesions with a dense lymphoplasmacytic infiltrate rich in IgG4 plasma cells and storiform-pattern fibrosis (2). The systemic disease affects almost all organs. Although there are some exceptions such as IgG4-related lymphadenopathy or the membranous glomerulonephritis secondary to IgG4-RD, the histopathologic findings generally show marked similarities across affected organs. The designation IgG4-related ophthal-mic disease (IgG4-ROD) was proposed when referring to the ophthalmic manifestations of IgG4-RD, with more specific terms applied with involvement of the lacrimal glands, extra-ocular muscles, and other orbital structures (Table 1). The diagnostic criteria for IgG4-RD are not yet firmly established nor are those for IgG4-ROD. The unifying features linking the disparate manifestations of IgG4-RD include characteristic histopathologic appearance and elevated number of IgG4+ plasma cells within organ tissue (3) (see table 1 in Ref. 1). Increased numbers of infiltrating IgG4-bearing plasma cells within involved organs are the sine qua non of the diagnosis. Although IgG4+/IgG+ plasma cell ratio in tissue .40% is generally accepted for diagnosis, there are some discordance in cutoff values of IgG4+ plasma cells. Comprehensive diagnos-tic criteria for IgG4-RD proposed by the Japanese IgG4-RD study groups set IgG4+ plasma cell counts .10 cells/high power field (HPF: magnification ·400) along with the IgG4+/IgG+ ratio .40% in the affected tissue (4). Both IgG4+ cell numbers and IgG4+/IgG+ cell ratio are required for diagnosis. Elevated serum IgG4 titers (.135 mg/dL) are not a diagnostic requirement as levels can be normal in 40% of patients with biopsy-proven IgG4-RD (5). The Japanese study group on IgG4-ROD reported the prevalence of IgG4-ROD based on either the number of IgG4+ plasma cells .30/HPF or a ratio of IgG4+/IgG+ cells .40% (6). In contrast, the Massachusetts General Hospital Center for IgG4-RD applied a cutoff of .100 IgG4+ Department of Visual Sciences and Ophthalmology, Faculty of Health and Medical Sciences, Aichi Shukutoku University, Aichi, Japan. The authors report no conflicts of interest. Address correspondence to Satoshi Kashii, MD, PhD, Department of Visual Sciences and Ophthalmology, Faculty of Health and Medical Sciences, Aichi Shukutoku University, Nagakute Campus, Aichi 480-1197, Japan. 400 Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 State-of-the-Art Review Section Editors: Valérie Biousse, MD Steven Galetta, MD Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. plasma cells/HPF for IgG4-ROD of the lacrimal glands (7). This seems based on a single study of 6 cases with chronic sclerosing dacryoadenitis (8). Deschamps et al (9) have ques-tioned the validity of the .100 cells per HPF threshold, indicating that a significant number of previously reported cases of IgG4-related orbital inflammation fell short of this threshold and that the variability of the cutoff value reflected heterogeneity of immunostaining for IgG4, the variability of selected sites for counting and sizes of HPF. The Japanese study group on IgG4-ROD now advocates a ratio of IgG4+/ IgG+ cells .40% and the number of IgG4+ cells .50 cells/ HPF (10). To further complicate matters, typical histologic findings, such as storiform-type fibrosis and obliterative phlebitis may be absent in lacrimal gland involvement. Currently, it seems prudent when making the diagnosis of IgG4-ROD to use the proposed 3-tiered diagnostic termi-nology for IgG4-RD (3) (see table 1 in Ref. 1). Table 2 summarizes the demographic data of 6 large case series of patients with IgG4-ROD. Although male predominance is characteristic of IgG4-RD, there is no gender predilection in IgG4-ROD (15). Bronchial asthma and allergic rhinitis are systemic conditions frequently asso-ciated with IgG4-ROD (16,17). LACRIMAL GLAND INVOLVEMENT The lacrimal gland is the most common site of IgG4-ROD and usually affected bilaterally. Yamamoto et al (18) first reported elevated serum IgG4 in patients with Mikulicz disease, characterized by symmetrical swelling of the lacrimal and/or major salivary glands. From studies con-ducted by the Japanese Society of Sjörgen syndrome (SjS) (19,20), it has become clear that SjS and Mikulicz disease are different clinical entities (see table 2 in Ref. 1). The most distinguishing feature lies in the pathological appear-ance of Mikulicz disease characterized by marked infiltra-tion of IgG4+ plasma cells, with a ratio of IgG4+ of .40%. This is rarely seen in patients with SjS. In contrast to SjS, Mikulicz disease is not associated with antinuclear antibody or anti-Ro or anti-La antibodies (21). Mikulicz disease had not been regarded as a systemic disease until reports docu-mented its association with extraglandular disorders, includ-ing allergic rhinitis, interstitial pneumonia, autoimmune pancreatitis, and interstitial nephritis (16). In a report of 44 patients with Mikulicz disease, approximately one-half had distant lesions consistent with IgG4-RD (22). IDIOPATHIC ORBITAL INFLAMMATION AND IgG4-RELATED ORBITAL DISEASE Sato et al (23) conducted the first large review of 112 patients with ocular adnexal lymphoproliferative disorders diagnosed between 1990 and 2006 and identified 21 cases of IgG4-orbital disease using the stored tissue samples. The diagnostic criterion used was 10 IgG4+ plasma cells per HPF. Of the 21 patients, 17 had lacrimal gland involve-ment with bilateral involvement in 12. Obliterative phlebi-tis was detected in only 2 patients, possibly indicating that obliterative phlebitis was organ-specific and not a clinical feature of IgG4-RD. In another study of archived paraffin-embedded tissues of orbital biopsy specimens, 21 patients were diagnosed with idiopathic orbital inflammation or reactive lymphoid hyperplasia (RLH) of which 11 (54%) had increased IgG4 cells (.10/HPF) (24). Unique clinical TABLE 1. Individual organ manifestations of IgG4-related ophthalmic disease Lacrimal glands IgG4-related dacryoadenitis Orbital soft tissue (orbital inflammatory pseudotumor) IgG4-related orbital inflammation (or IgG4- related orbital inflammatory pseudotumor) Extraocular muscles IgG4-related orbital myositis Orbit with involvement of multiple anatomic structures IgG4-related panorbital inflammation (includes lacrimal gland disease, extraocular muscle involvement, and other potential intraorbital complications) IgG4, immunoglobuolin G4. TABLE 2. Case series of IgG4-related ophthalmic disease Report Number of Patients Gender (Male/ Female) Age Range, yr (Mean) Scope of Report Takahira et al (11) 16 8/8 41-76 (56) IgG4-related orbital inflammation Sato et al (12) 21 10/11 39-86 (59) Ocular adnexal IgG4-related disease Kubota et al (13) 10 5/5 38-73 (58) Ocular adnexal IgG4-related disease Go et al (14) 14 7/7 20-72 (51) Ocular adnexal IgG4-related disease Japanese study group (6) 219 105/114 23-90 (61) IgG4-related orbital inflammation Wallace et al (7) 21 12/9 24-79 (50) IgG4-related ophthalmic disease IgG4, immunoglobuolin G4. Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 401 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. features of the IgG4 patients included eyelid swelling, bilat-eral orbital involvement, other organ involvement (pan-creas, biliary tract, and liver), and a prolonged clinical course. A history of asthma was noted in 5 patients. In another retrospective study using archived biopsied speci-mens, 10 of 25 (40%) patients with idiopathic orbital inflammation fulfilled both criteria .10 IgG4+ plasma cells per HPF and ratios of IgG4+/IgG plasma cells .40%. Only 2 of 10 patients with a positive biopsy for IgG4 had bilateral involvement, and no distinctive systemic features in the IgG4+ patients were detected compared with IgG4- negative patients. Neuroimaging of IgG4-ROD reveals orbital lesions with soft tissue attenuation and well-defined margins on precon-trast CT, isosignal intensity on T1-weighted magnetic resonance imaging (MRI), hypointensity of T2-weighted scans reflecting increased cellularity and fibrosis, and a homogenous enhancement pattern after intravenous contrast (25). Lesions usually are not destructive, and bone adjacent to the lesions demonstrates remodeling without destruction (26). However, there are case reports of destruc-tive bone involvement in biopsy-proven IgG4-RD, espe-cially in cases affecting the paranasal sinuses (7,27-29). IgG4-RD involving the nasal cavity and paranasal sinuses also may show perineural and bone marrow infiltration mimicking malignant lymphoma (30). In an orbital imaging study of 65 patients with IgG4- related orbital disease by Sogabe et al (31), lacrimal gland involvement was present in 56 patients (88%) of these 56 patients, it was isolated in 31 and bilateral in 52. Isolated lacrimal gland enlargement and trigeminal nerve enlarge-ment were seen in 49% (31/65). After lacrimal gland enlargement, (40%, 25/65), extraocular myositis (25%, 16/65) and compressive optic neuropathy (9%, 6/65) were the most frequent imaging findings. There are some clinical features that distinguish IgG4-related orbital disease from idiopathic orbital inflammation (Table 3). EXTRAOCULAR MUSCLE INVOLVEMENT Extraocular muscle involvement has not been reported in isolation but rather accompanied by involvement of the lacrimal gland, infra-orbital nerve, and/or surrounding orbital soft tissue. Sogabe et al (31) found no predilection for any particular ex-traocular muscle in 16 patients with IgG4-related orbital dis-ease. Only 3 of the 16 patients had restricted eye movements. The disparity between the enlargement of extraocular muscles on CT and relatively spared eye movements seems suggestive of IgG4-related orbital myositis. In a case of biopsy-confirmed IgG4-related orbital myositis, the lateral rectus muscle was dif-fusely expanded with an inflammatory infiltrate composed of lymphoid follicles and abundant plasma cells of which .90% were IgG4+ (32). On occasion, it may be difficult to differen-tiate thyroid eye disease from IgG4-related orbital disease (33). TWO SUBTYPES OF IgG4-ROD In contrast to IgG4-ROD primarily affecting the lacrimal gland,Mehta et al (34) reported a unique case of IgG4-related orbital sclerosing pseudotumor involving the orbital periosteum TABLE 3. IgG4-related orbital disease versus idiopathic orbital inflammatory syndrome IgG4-related Orbital Disease Idiopathic Orbital Inflammation Mode of Onset Chronic Acute, subacute, chronic Pain No Often present, may be severe Laterality Usually bilateral Usually unilateral Lacrimal gland enlargement Usually with salivary gland involvement Isolated dacryoadenitis Infraorbital nerve enlargement Frequent (pathognomonic) Extremely rare Orbital myositis Multimuscle involvement Often single (or 2) muscle inflammation No particular mode MR . SR . LR . IR Relatively spared eye movements Restriction of eye movement in the field of action of the affected muscles Always accompanied by other orbital lesions Usually isolated Optic nerve involvement Compressive optic neuropathy Optic perineuritis Associated systemic diseases Allergic rhinitis; asthma Rare Other organ involvement Lymphadenopathy None Autoimmune pancreatitis type I Sclerosing cholangitis Retroperitoneal fibrosis IgG4, immunoglobuolin G4; LR, lateral rectus; IR, inferior rectus; MR, medical rectus; SR, superior rectus. 402 Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. preferentially and sparing the lacrimal glands. Biopsy of the orbital mass revealed a classic histopathologic picture of idio-pathic sclerosing inflammation with scattered lymphoid aggre-gates without germinal center. IgG4-positive plasma cells (.35 per HPF) were identified in the lymphoid clusters. The authors proposed 2 types of IgG4-ROD (Table 4). NON-IgG4-ROD WITH ELEVATED NUMBER OF IgG4+ CELLS The consensus statement on Pathology of IgG4-RD (3) listed a number of conditions that fall outside the IgG4-RD spec-trum, which may be associated with increased number of IgG4+ plasma cells. This includes anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, Rosai-Dorfman dis-ease, and multicentric Castleman disease. It remains unsettled whether there is a xanthogranulomatous variant of IgG4- ROD. Orbital xanthogranulomas is a histiocytic disorder of non-Langerham cells (class II) and divided into 4 categories: adult-onset xanthogranuloma (AOX) typically not associated with systemic lesions, adult-onset asthma and periocular xanthogranulomatous (AAPOX), necrobiotic xanthogranuloma, and Erdheim-Chester disease. Sivak-Callcot et al (35) have shown that xanthogranulomatous lesions are localized to the anterior preseptal portion of the orbit. Some reports suggest that xanthogranulomatous cases are a variant of IgG4-ROD (36,37), whereas others contend that AOX and AAPOX may be manifestations of IgG4-ROD (7). TRIGEMINAL NERVE INVOLVEMENT Trigeminal nerve branch enlargement is a unique clinical feature of IgG4-related orbital disease. Ohshima et al (38) first called attention to infra-orbital nerve enlargement (see figure 2 in Ref. 1), as a useful clinical sign for IgG4-related orbital disease based on their case series study of 71 patients with orbital lymphoproliferative disorders. Subsequent reports have confirmed these findings (26,39). Sogabe et al (31) docu-mented this in 25 of 65 (40%) of these patients, with bilateral involvement in 16 of 25 (40%). Interestingly, none of the patients with trigeminal nerve enlargement complained of sensory disturbances. Hardy et al (40) examined IgG4- positivity of stored orbital biopsy samples in 14 patients including 6 specimens with infra-orbital nerve lesions. Immu-nophenotypic compatibility was based on an absolute IgG4 cell count of .10 cells per HPF. Seven specimens fulfilled this criterion, whereas the rest were categorized as RLH. All cases of infra-orbital nerve enlargement also had extraocular muscle enlargement and sinus disease. Two-thirds also had lacrimal gland enlargement. Inflammatory infiltration of the trigeminal epineurium was present in all 6 biopsy specimens, whereas the endoneurium and perineurium were unaffected. The lack of involvement of the perineurium or nerve fibers of the trigem-inal nerve may explain the lack of sensory symptoms. OPTIC NERVE INVOLVEMENT Sixteen patients with optic nerve involvement have been reported in the literature (age range, 28-73; male/female ratio: 13/3) (8,24,31,32,41) (Fig. 1). Presumed causes of the optic neuropathy include infra-orbital nerve enlarge-ment compressing the optic nerve in the orbital apex in 5 patients, a localized orbital mass surrounding the optic nerve in 5 patients, extraocular muscle enlargement in the orbital apex in 3 patients, a diffuse orbital fat lesion sur-rounding the optic nerve in 2 patients, and localized orbital mass compressing the optic nerve in 1 patient. In 4 patients, Inoue et al (41) demonstrated that IgG4-related compres-sive optic neuropathy was steroid-responsive including 1 patient with recurrence during steroid taper that promptly responded to an increased dose of steroids. On MRI, the lesion was reduced in size but was still present. It is possible that the optic neuropathy may be due to an infiltrative or inflammatory process, given the observation of perineural spread of IgG4 plasma cells along the trigeminal nerve. LYMPHOMA COMPLICATING IGG4-ROD VERSUS DE NOVO LYMPHOMA According to the prevalence study of the Japanese multi-center study group (6), IgG4-ROD accounts for about a quarter of orbital lymphoproliferative disorders in Japan. TABLE 4. Two subtypes of IgG4-related ophthalmic disease Site Chronic Sclerosing Dacryoadenitis Classic Sclerosing Orbital Pseudotumor Lacrimal Glands Periorbital Membrane Lacrimal gland involvement Always present No Orbital involvement Bilateral Unilateral Main histopathologic finding Reactive lymphoid hyperplasia with a component of reactive fibrosis Fibrosclerosis with scattered lymphoid aggregates Lymphoid follicular formation Marked No Related pathology IgG4+-related ophthalmic disease IgG4+ sclerosing pancreatitis IgG4+ retroperitoneal fibrosis Potential evolution into lymphoma Yes (MALT and follicular lymphoma) No IgG4, immunoglobuolin G4. Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 403 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. In that report, 44 of 448 (9.8%) cases of extranodal mar-ginal B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type were found to have IgG4+ plasma cell infiltrates. In contrast to IgG4-RD in other organs, the extensive morphologic overlap with reactive lesions and low-grade B-cell lymphoma has been described in IgG4- related orbital disease (32). Cheuk et al (42) first reported 6 patients with ocular adnexal lymphoma associated with IgG4-related chronic dacryoadenitis. Monoclonal B-cell proliferation, as demonstrated by the presence of monoclo-nal immunoglobulin heavy chain gene rearrangement or light chain restriction, was documented in 2 of 17 (12%) patients (14) and 2 of 14 (14.3%) (23) patients with IgG4- related orbital disease. A number of case series have raised the issue whether MALT lymphomas develop from IgG4- related orbital inflammation or IgG4-producing cells are capable of transforming to de novo IgG4-orbital MALT lymphoma (12,43-45). DIFFERENTIAL DIAGNOSES OF IgG4-ROD ANCA-associated vasculitis may affect ocular adnexa and create a clinical appearance of idiopathic orbital inflammation accompanied by hyper-IgG4 gammaglobulinemia and increased number of IgG4 plasma cells. ANCA vasculidities include microscopic polyangiitis, granulomatosis with poly-angiitis (formerly known as Wegener granulomatosis), and eosinophilic granulomatosis with polyangitis (formerly known as Churg-Strauss Syndrome). These lesions are char-acterized by the presence of granulomatous inflammation with vasculitis and necrosis. Periocular pain can differentiate these patients from those with IgG4-related orbital disease who do not complain of pain. Multicentric Castleman disease has been recognized to exhibit hyper-IgG4 gammaglobulinemia and IgG4+ plasma cell infiltration with fibrosclerosis of tissue. In such cases, measurement of the serum interleukin-6 has been recom-mended to differentiate Castleman disease from IgG4- ROD and to determine whether corticosteroids should be initiated (16). OTHER IgG4-RD OF NEURO-OPHTHALMOLOGIC INTEREST Central nervous system involvement of IgG4-RD has been described in the form of either hypophysitis (46) or hyper-trophic cranial pachymeningitis (47). The pituitary gland and stalk are known to be involved in IgG4-related systemic disease (48). The first pathologi-cally proven case of IgG4-related hypophysitis was a 77- year-old man with a medical history of autoimmune pancreatitis and sclerosing cholangitis who reported visual failure over several months (49). He was found to have optic atrophy due to a suprasellar mass. Transsphenoidal resection revealed a histopathology compatible with IgG4- RD. Immunohistochemical study also showed IgG4+ plasma cell infiltration in the pancreatic biopsy and chole-cystectomy specimen. Thirty-five cases of IgG4-related hypophysitis have been reported based on the presence of pituitary and stalk lesions associated with high serum IgG4 levels and/or histopathologic findings of pituitary biopsy (50,51). Pituitary biopsies were performed in 14 cases. Twelve biopsies showed IgG4+ plasma cell infiltration. Twenty-nine cases (83%) were men, and ages ranged from the third to ninth decade (mean: 65.7 years). In contrast to lymphocytic hypophysitis that primarily affects women, IgG4-related hypophysitis seems to develop in older men. Twenty-five patients presented with hypopituitarism and 21 with diabetes insipidus. In two-thirds of cases, both pituitary and stalk enlargement (infundibulo-hypophysitis) were observed on neuroimaging. Five patients (14%) had isolated hypophysitis without any other lesions. One of them presented bitemporal hemianopia that was the sole clinical manifestation of the biopsy-proven IgG4-related hypophysitis (52). Thirty patients had other IgG4-related systemic diseases, including pulmonary lesions, retroperitoneal fibrosis, autoimmune pan-creatitis, lymph node swelling, salivary and/or lacrimal glands lesions, pachymeningitis, and kidney lesions. Of note, Shimatsu FIG. 1. IgG4-related compressive optic neuropathy. T2 axial magnetic resonance imaging (A) and postcontrast T1 coro-nal MRI with fat suppression (B) show compression of the left optic nerve by surrounding soft tissue mass and enlarged extraocular muscles (Courtesy of H. Tsuji, MD and Y. Sogabe, MD). 404 Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. et al (53) found that in patients with IgG4-related hypophysitis there was an association with pachymeningitis, sinusitis, and/or orbital inflammation. Lymphocytic hypophysitis is considered to rarely spread to the surrounding structures in contrast to IgG4-related hypophysitis (54). Hypertrophic pachymeningitis is a chronic fibrosing inflammatory disorder causing diffuse or localized thickening of the cranial and spinal cord dura mater. According to a recent nationwide survey of hypertrophic pachymeningitis (n = 159) in Japan (55), headache was the most common initial symptoms (56/159, 35%), followed by ophthalmolog-ical symptoms, such as visual loss (21/159, 14%), and diplopia (20/159, 13%). Known causes included ANCA-associated vasculitis (48/159, 30%) and IgG4-RD (14/159, 9%). Six additional patients with ANCA-associated vasculitis also showed either hyper-IgG4 gammaglobulinemia or increased number of IgG4+ plasma cells in the biopsied dura. IgG4- related hypertrophic pachymeningitis (IgG4-RHP, n = 14) affected predominantly men, whereas ANCA-RHP preferen-tially affected women. There was no difference in age at onset between IgG4-RHP (56.7 ± 12.5 years) and ANCA-RHP (62.5 ± 14.4 years). All IgG4-RHP patients had cranial pachymeningitis, and none presented with isolated hypertro-phic spinal pachymeningitis. Otological symptoms and elevated systemic inflammatory markers (erythrocyte sedimentation rate, C-reactive protein, white blood cell count) were more frequently noted in patients with ANCA-RHP, whereas IgG4-RHP showed higher frequency of diplopia (P = 0.0149). It has been suggested that some patients diag-nosed with Tolosa-Hunt syndrome on clinical grounds alone actually may have IgG4-RD (7,47). When active lesions in patients with IgG4-RHP are in close proximity to the blood-brain barrier and cerebrospinal fluid (CSF) circulation, serum IgG4 levels may remain nor-mal. Lu et al (56) proposed that elevated serum IgG4 levels in patients with IgG4-RHP indicate extrameningeal involve-ment. CSF examination in patients with IgG4-RHP gener-ally shows a lymphocytic pleocytosis with a variable degree of protein elevation. Della-Torre et al (57) have evaluated the intrathecal production of total IgG and IgG subclasses using IgG index (CSF IgG/serum IgG O serum albumin/ CSF albumin) and IgGLOC values in patients with other causes of hypertrophic pachymeningitis. These investigators proposed that CSF IgG4 levels (.2.27 mg/dL) and IgG4Loc (.0.47) may be helpful for diagnosing IgG4-RHP. Radio-logically, fibrotic hypertrophihc dura is iso- to hypo-intense on T1-weightedMRI and hypointense on T2-weighted images with occasional scattered foci of hyperintensity suggestive of inflammation (56). Gadolinium-enhanced MRI demonstrates intense dural enhancement in a linear or nodular pattern reflecting a linear dural thickening or a bulging mass. Wallace et al (47) noted that nonspecific scattered white matter T2 hyperintensities were observed in aged patients with ANCA-associated vasculitis, rheumatoid arthritis, neurosarcoidosis, and lymphoma but not in patients with IgG4-RD. The standard first-line treatment for IgG4-RHP is systemic corticosteroids followed by the addition of other immunosuppressants such as methotrexate, azathioprine, mycophenolate mofetil, and cyclophosphamide in the event of a recurrence (56). Rituximab is the most promising emerging steroid-sparing agent for IgG4-RD, but the pub-lished clinical experience of rituximab in patients with IgG4-RHP is limited (47) (56). TREATMENT OF IgG4-ROD At present, there are no evidence-based treatment guide-lines for either IgG4-ROD or IgG4-RD. Rather, treatment should be individualized. Some cases of IgG4-ROD may show spontaneous remission (23). Generally, IgG4-ROD is characterized by a marked response to corticosteroid early in the disease course. However, IgG4 disease has been often known to recur during steroid taper and often requires long-term therapy. Using rituximab, anti-CD20+ monoclonal antibody, Khosroshahi et al (58) succeeded in discontinuing system steroids without relapse in 10 con-secutive patients with steroid-refractory IgG4-RD. In a meta-analysis of definite and probable cases of IgG4- ROD (n = 59), men and women showed equal resistance to 1 or more treatment modalities (15). Further data on treatment of IgG4-ROD will become available with pub-lication of ongoing clinical trials (7). REFERENCES 1. Yamamoto M, Masato H, Takahashi H, Shinomura Y. IgG4-Related disease. J Neuroophthalmol. 2014;34:393-399. 2. Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, Azumi A, Bloch DB, Brugge WR, Carruthers MN, Cheuk W, Cornell L, Castillo CF, Ferry JA, Forcione D, Klöppel G, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Masaki Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani D, Sato Y, Smyrk T, Stone JR, Takahira M, Umehara H, Webster G, Yamamoto M, Yi E, Yoshino T, Zamboni G, Zen Y, Chari S. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061-3067. 3. Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Klöppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181-1192. 4. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Nakamura S, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD) 2011. Mod Rheumatol. 2012;22:21-30. 5. Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011;23:108-113. 6. Japanese Study Group of IgG4-Related Ophthalmic Disease. A prevalence study of IgG4-related ophthalmic disease in Japan. Jpn J Ophthalmol. 2013;57:573-579. Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 405 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 7. Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 2014;43:806-817. 8. Cheuk W, Yuen HK, Chan JK. Chronic sclerosing dacryoadenitis: part of the spectrum of IgG4-related sclerosing disease? Am J Surg Pathol. 2007;31:643-645. 9. Deschamps R, Deschamps L, Depaz R, Coffin-Pichonnet S, Belange G. High prevalence of IgG4-related lymphoplasmacytic infiltrative disorder in 25 patients with orbital inflammation: a retrospective case series. Br J Ophthalmol. 2013;97:999-1004. 10. Goto H, Takahira M, Azumi A. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn J Ophthalmol. In press. doi: 10.1007/ s10384-014-0352-2. 11. Takahira M, Ozawa Y, Kawano M, Zen Y, Hamaoka S, Yamada K, Sugiyama K. Clinical aspects of IgG4-related orbital inflammation in a case series of ocular adnexal lymphoproliferative disorders. Int J Rheumatol. 2012;2012:635473. 12. Sato Y, Notohara K, Kojima M, Takata K, Masaki Y, Yoshino T. IgG4-related disease: historical overview and pathology of hematological disorders. Pathol Int. 2010;60:247-258. 13. Kubota T, Moritani S, Katayama M, Terasaki H. Ocular adnexal IgG4-related lymphoplasmacytic infiltrative disorder. Arch Ophthalmol. 2010;128:577-584. 14. Go H, Kim JE, Kim YA, Chung HK, Khwarg SI, Kim CW, Jeon YK. Ocular adnexal IgG4-related disease: comparative analysiswith mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology. 2012;60:296-312. 15. Andrew N, Kearney D, Selva D. IgG4-related orbital disease: a meta-analysis and review. Acta Ophthalmol. 2013;91:694-700. 16. Masaki Y, Dong L, Kurose N, Kitagawa K, Morikawa Y, Yamamoto M, Takahashi H, Shinomura Y, Imai K, Saeki T, Azumi A, Nakada S, Sugiyama E, Matsui S, Origuchi T, Nishiyama S, Nishimori I, Nojima T, Yamada K, Kawano M, Zen Y, Kaneko M, Miyazaki K, Tsubota K, Eguchi K, Tomoda K, Sawaki T, Kawanami T, Tanaka M, Fukushima T, Sugai S, Umehara H. Proposal for a new clinical entity, IgG4- positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis. 2009;68:1310-1315. 17. Matsui S, Taki H, Shinoda K, Suzuki K, Hayashi R, Tobe K, Tokimitsu Y, Ishida M, Fushiki H, Seto H, Fukuoka J, Ishizawa S. Respiratory involvement in IgG4-related Mikulicz's disease. Mod Rheumatol. 2012;22:31-39. 18. Yamamoto M, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Takahashi H, Imai K. Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scand J Rheumatol. 2004;33:432-433. 19. Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, Tsubota K, Yoshino T, Kawa S, Suzuki R, Takegami T, Tomosugi N, Kurose N, Ishigaki Y, Azumi A, Kojima M, Nakamura S, Inoue D. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1-14. 20. Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz's disease and sclerosing pancreatitis: diagnostic insights. J Rheum. 2010;37:1380-1385. 21. Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz's diseaseas anIgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335-340. 22. Himi T, Takano K, Yamamoto M, Naishiro Y, Takahashi H. A novel concept of Mikulicz's diseaseas IgG4-related disease. Auris Nasus Larynx. 2012;39:9-17. 23. Sato Y, Ohshima K, Ichimura K, Sato M, Yamadori I, Tanaka T, Takata K, Morito T, Kondo E, Yoshino T. Ocular adnexal IgG4- related disease has uniform clinicopathology. Pathol Int. 2008;58:465-470. 24. Plaza JA, Garrity JA, Dogan A, Ananthamurthy A, Witzig TE, Salomão DR. Orbital inflammation with IgG4-positive plasma cells. Arch Ophthalmol. 2011;129:421-428. 25. Song YS, Choung HK, Park SW, Kim JH, Khwarg SI, Jeon YK. Ocular adnexal IgG4-related disease: CT and MRI findings. Br J Ophthalmol. 2013;97:412-418. 26. Katsura M, Mori H, Kunimatsu A, Sasaki H, Abe O, Machida T, Ohtomo K. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology. 2012;54:873-882. 27. Ishida M, Hotta M, Kushima R, Shibayama M, Shimizu T, Okabe H. Multiple IgG4-related sclerosing lesions in the maxillary sinus, parotid gland and nasal septum. Pathol Int. 2009;59:670-675. 28. Pace C, Ward S. A rare case of IgG4-related sclerosing disease of the maxillary sinus associated with bone destruction. J Oral Maxillofac Surg. 2010;68:2591-2593. 29. Alt JA, Whitaker GT, Allan RW, Vaysberg M. Locally destructive skull base lesion: IgG4-related sclerosing disease. Allergy Rhinol (Providence). 2012;3:e41-e45. 30. Sasaki T, Takahashi K, Mineta M, Fujita T, Aburano T. Immunoglobulin G4-related sclerosing disease mimicking invasive tumor in the nasal cavity and paranasal sinuses. AJNR Am J Neuroradiol. 2012;33:19-20. 31. Sogabe Y, Ohshima K, Azumi A, Takahira M, Kase S, Tsuji H, Yoshikawa H, Nakamura T. Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol. 2014;252:531-538. 32. Karamchandani JR, Younes SF, Warnke RA, Natkunam Y. IgG4- Related systemic sclerosing disease of the ocular adnexa: a potential mimic of ocular lymphoma. Am J Clin Pathol. 2012;137:699-711. 33. Inaba H, Hayakawa T, Miyamoto W, Takeshima K, Yamaoka H, Furukawa Y, Kawashima H, Ariyasu H, Wakasaki H, Furuta H, Nishi M, Nakao T, Sasaki H, Okada Y, Matsunaga K, Nakamura Y, Akamizu T. IgG4-related ocular adnexal disease mimicking thyroid-associated orbitopathy. Intern Med. 2013;52:2545-2551. 34. Mehta M, Jakobiec F, Fay A. Idiopathic fibroinflammatory disease of the face, eyelids, and periorbital membrane with immunoglobulin G4-positive plasma cells. Arch Pathol Lab Med. 2009;133:1251-1255. 35. Sivak-Callcott JA, Rootman J, Rasmussen SL, Nugent RA, White VA, Paridaens D, Currie Z, Rose G, Clark B, McNab AA, Buffam FV, Neigel JM, Kazim M. Adult xanthogranulomatous disease of the orbit and ocular adnexa: new immunohistochemical findings and clinical review. Br J Ophthalmol. 2006;90:602-608. 36. Singh K, Rajan KD, Eberhart C. Orbital necrobiotic xanthogranuloma associated with systemic IgG4 disease. Ocul Immunol Inflamm. 2010;18:373-378. 37. Mudhar HS, Bhatt R, Sandramouli S. Xanthogranulomatous variant of immunoglobulin G4 sclerosing disease presenting as ptosis, proptosis and eyelid skin plaques. Int Ophthalmol. 2011;31:245-248. 38. Ohshima K, Sogabe Y, Sato Y. The usefulness of infraorbital nerve enlargement on MRI imaging in clinical diagnosis of IgG4-related orbital disease. Jpn J Ophthalmol. 2012;56:380-382. 39. Katsura M, Morita A, Horiuchi H, Ohtomo K, Machida T. IgG4- related inflammatory pseudotumor of the trigeminal nerve: another component of IgG4-related sclerosing disease? AJNR Am J Neuroradiol. 2011;32:E150-E152. 40. Hardy TG, McNab AA, Rose GE Enlargement of the infraorbital nerve: an important sign associated with orbital reactive lymphoid hyperplasia or immunoglobulin g4-related disease. Ophthalmology. 2014;121:1297-1303. 41. Inoue D, Zen Y, Sato Y, Abo H, Demachi H, Uchiyama A, Gabata T, Matsui O. IgG4-related perineural disease. Int J Rheumatol. 2012;2012:401890. 42. Cheuk W, Yuen HK, Chan AC, Shih LY, Kuo TT, Ma MW, Lo YF, Chan WK, Chan JK. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. Am J Surg Pathol. 2008;32:1159-1167. 43. Kubota T, Moritani S, Yoshino T, Nagai H, Terasaki H. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4- positive plasma cells. J Clin Pathol. 2010;63:1059-1065. 44. Sato Y, Ohshima K, Takata K, Huang X, Cui W, Ohno K, Yoshino T. Ocular adnexal IgG4-producing mucosa-associated lymphoid tissue lymphoma mimicking IgG4-related disease. J Clin Exp Hematop. 2012;52:51-55. 406 Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 45. Oyama T, Takizawa J, Nakamura N, Aoki S, Aizawa Y, Abe H. Multifocal mucosa-associated lymphoid tissue lymphoma associated with IgG4-related disease: a case report. Jpn J Ophthalmol. 2011;55:304-306. 46. van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Ann Intern Med. 2004;141:896-897. 47. Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, Deshpande V, Stone JH. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013;92:206-216. 48. Leporati P, Landek-Salgado MA, Lupi I, Chiovato L, Caturegli P. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab. 2011;96:1971-1980. 49. Wong S, Lam WY, Wong WK, Lee KC. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol. 2007;38:1720-1723. 50. Ohkubo Y, Sekido T, Takeshige K, Ishi H, Takei M, Nishio S, Yamazaki M, Komatsu M, Kawa S, Suzuki S. Occurrence of IgG4- related hypophysitis lacking IgG4-bearing plasma cell infiltration during steroid therapy. Intern Med. 2014;53:753-757. 51. Iseda I, Hida K, Tone A, Tenta M, Shibata Y, Matsuo K, Yamadori I, Hashimoto K. Prednisolone markedly reduced serum IgG4 levels along with the improvement of pituitary mass and anterior pituitary function in a patient with IgG4-related infundibulo-hypophysitis. Endocr J. 2014;61:195-203. 52. Hattori Y, Tahara S, Ishii Y, Kitamura T, Inomoto C, Osamura RY, Teramoto A, Morita A. A case of IgG4-related hypophysitis without pituitary insufficiency. J Clin Endocrinol Metab. 2013;98:1808-1811. 53. Shimatsu A, Oki Y, Fujisawa I, Sano T. Pituitary and stalk lesions (infundibulo-neurohypophysitis) associated with immunoglobulin IgG4-related systemic disease: an emerging clinical entity. Endocr J. 2009;56:1033-1041. 54. Kanoke A, Ogawa Y, Watanabe M, Kumabe T, Tominaga T. Autoimmune hypophysitis presenting with intracranial multi-organ involvement: three case reports and review of the literature. BMC Res Notes.2013;6:560. 55. Yonekawa T, Murai H, Utsuki S, Matsushita T, Masaki K, Isobe N, Yamasaki R, Yoshida M, Kusunoki S, Sakata K, Fujii K, Kira J. A nationwide survey of hypertrophic pachymeningitis in Japan. J Neurol Neurosurg Psychiatry. 2014;85:732-739. 56. Lu LX, Della-Torre E, Stone JH, Clark SW. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol. 2014;71:785-793. 57. Della-Torre E, Galli L, Franciotta D, Bozzolo EP, Briani C, Furlan R, Roveri L, Sessa M, Passerini G, Sabbadini MG. Diagnostic value of IgG4 Indices in IgG4-related hypertrophic pachymeningitis. J Neuroimmunol. 2014;266:82-86. 58. Khosroahahi A, Carruthers NM, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore). 2012;91:57-66. Kashii: J Neuro-Ophthalmol 2014; 34: 400-407 407 State-of-the-Art Review Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |