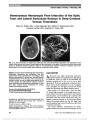
| OCR Text |
Show Peripheral Homonymous Hemianopia: Correlation Between Lesion Location and Visual Field Defects by Means of Cytoarchitectonic Probabilistic Maps Eleni Papageorgiou, MD, Luca F. Ticini, PhD, Ulrich Schiefer, MD Background: Peripheral homonymous scotomas beyond 30 from fixation are rare. The paucity of publications describing such visual field defects might be attributed to various factors, including the absence of severe symp-toms, routine visual field assessment restricted to the central 30 with automated perimetry, and the collateral circulation to the occipital cortex. The aim of this study was to correlate the brain lesions and perimetric findings in 2 unusual cases of peripheral homonymous scotomas, with the anatomic location of the optic radiation and primary visual cortex. Methods: Two patients with circumscribed homonymous scotomas beyond 30 related to infarcts in the in-termediate area of the visual cortex are reported. We describe a new strategy, which relies on modern lesion analysis and stereotaxic probabilistic cytoarchitectonic maps, to accurately correlate the brain lesion site with the location of the peripheral homonymous visual field defects. Results: In Case 1, the posterior optic radiation was af-fected in its termination in the upper intermediate visual cortex. In Case 2, the lesion was located in the upper rostral portion of the primary visual cortex. In both, the most anterior part of the visual cortex and the occipital pole were intact, accounting for preservation of the central and most peripheral visual field. Additionally, correlation of the neuroimaging findings with commonly used maps of the representation of the visual field on the striate cortex suggested that our data were most con-sistent with the Holmes map. Conclusions: Modern lesion analysis and cytoarchitec-tonic maps, in combination with the existing retinotopic maps, may provide reliable clues for the localization of cerebral infarction and prognosis of homonymous visual field defects and may lead to a better understanding of the link between neuroanatomical landmarks and functional outcomes. Journal of Neuro-Ophthalmology 2012;32:5-12 doi: 10.1097/WNO.0b013e31821fc0e9 2012 by North American Neuro-Ophthalmology Society Peripheral homonymous visual field defects (HVFDs) sparing more than 10 of the vertical meridian are rare. As a consequence, retinotopic maps depict just a rough estimate of the peripheral visual field representation (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/WNO/A20) (1-3). Three documented cases have described homonymous scotomas beyond 25 from fixation. Mejico et al (4) reported a patient with a peripheral HVFD located 40-60 from fixation due to a cavernous angioma. A second patient with a right hom-onymous hemianopia beyond the central 50 due to an infarction in the ventral portion of the left calcarine fissure was described by Reche-Sainz et al (5). Barton and Benatar (6) reported a patient with a peripheral HVFD extending between 25 and 40 from fixation caused by an ischemic stroke in the midportion of the inferior calcarine cortex. According to the existing retinotopic maps, lesions affecting this portion of the visual field-from 30 to 60 -are located in the intermediate striate cortex (1-3,7). With the introduction of functional magnetic resonance imaging, a significant amount of data about the organization of human visual field maps has been produced and the posi-tion, surface area, and visual field representation of various intact human visual areas have been documented (8-12). Centre for Ophthalmology (EP, US), Institute for Ophthalmic Re-search, University of Tuebingen, Tuebingen, Germany; and Max Planck Institute for Human Cognitive and Brain Sciences (LFT), Leipzig, Germany. E.P. and L.F.T. contributed equally to this study. U.S. is a consultant of HAAG-STREIT, Inc, the manufacturer of the perimeter used in this study. Supported by the European Union (PERACT-Marie Curie Early Stage Training MEST-CT-2004-504321). The authors state that they have no proprietary interest in the products named in this article. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jneuro-ophthalmology.com). Address correspondence to Eleni Papageorgiou, MD, Centre for Ophthalmology, University of Tuebingen, Schleichstrasse 12-16, Tuebingen 72076, Germany; E-mail: e_papage@yahoo.com Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 5 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 1. Case 1. (A) 30 threshold-related, slightly supraliminal automated static perimetry. There are defect points (red circles) on the left outer border in both eyes. (B) Semiautomated kinetic perimetry of the binocular 90 visual field for stimulus III4e. A left inferior absolute homonymous peripheral scotoma is present, extending between 30 and 50 along the horizontal meridian. 6 Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. To gain further understanding of cortical representation of the visual field, we adopted a novel approach making use of lesion analysis techniques in combination with recently developed stereotaxic probabilistic cytoarchitectonic maps (13) of the optic radiation (14,15) and the primary visual cortex (16). We applied this method to investigate 2 new cases of peripheral homonymous scotomas beyond 30 . CASE REPORTS Case 1 A 39-year-old woman was admitted to the hospital for persistent visual phenomena in her left visual field after a migraine attack. On examination, the patient's best-corrected visual acuity was 20/20 in both eyes, with normal color vision and normal ocular motility. Pupils were equal in diameter and showed no relative afferent pupillary defect. Visual fields assessed with 30 threshold-related, slightly supraliminal automated static perimetry (Octopus 101 perimeter; HAAG-STREIT Inc., Koeniz, Switzerland) were normal except for 2 single-defect points on the left outer border that were present in both eyes (Fig. 1A). Binocular 90 semiautomated kinetic perimetry using a III4e stimulus revealed an absolute left inferior homonymous peripheral scotoma (Fig. 1B). CT revealed an ischemic infarction in the right occipital region. On further investigation, no cardiovascular risk factors were detected. Neuro-ophthal-mological examination 12 months later demonstrated that the left peripheral homonymous defect was unchanged from the initial examination. MRI acquired 12 months post-stroke was used to determine the extent of the lesion (Fig. 2A). The posterior extent of the lesion was located 15 mm from the occipital pole, from which the lesion extended 18 mm anteriorly and spared 12 mm of the most anterior aspect of the right calcarine fissure. Lesion Analysis The boundary of the lesion was delineated directly on each transverse slice of the individual MRI using MRIcro soft-ware (http://www.mricro.com) (17). Both the MRI images and the lesion shapes were then transformed into stereotaxic space using the spatial normalization algorithm provided by SPM5 (http://www.fil.ion.ucl.ac.uk/spm/), using default settings. For determination of the transformation parame-ters, cost-function masking was employed (18). The resulting normalized lesion area was plotted onto the stereotaxic cytoarchitectonic probabilistic maps of the optic radiation (Fig. 2B) and the primary visual cortex (Fig. 2C) (14-16). These maps illustrate the relative fre-quency (from 1 to 10) of appearance of a cytoarchi-tectonically labeled structure of interest in 10 adult human brains, which were normalized on the reference brain provided by SMP5 (e.g., a value of 5 for a specified structure in a certain voxel indicates that the structure was present in that location in 5 of 10 brains). The probabilistic cy-toarchitectonic maps thus served as a measure of inter-subject variability for each voxel in the reference space. By superimposing the lesion area onto the cytoarchitectonic maps, we found that the investigated scotoma was induced by a lesion affecting the posterior part of the optic radiation, at its termination in the upper intermediate visual cortex (Fig. 2B, C, Slice 16). The most anterior part of the visual cortex and the occipital pole were intact. Case 2 A 54-year-old man with a 25-year history of occipital headaches was admitted to the hospital following acute visual field loss on his right side after a severe episode of headache. His medical history was remarkable only for smoking. On examination, the best-corrected visual acuity was 20/20 in both eyes, pupils were equal in diameter and showed no relative afferent pupillary defect, and slit-lamp examination was normal. Visual field testing with 30 threshold-related, slightly supraliminal automated static perimetry (Octopus 101 perimeter) was normal except for a few defects on the right outer border of both eyes (Fig. 3A). Threshold-related, supraliminal automated static perimetry in the 90 visual field revealed absolute right inferior homonymous peripheral scotomas, extending from 30 to 60 in both eyes (Fig. 3B). The MRI images acquired 1 day after onset of symptoms demonstrated 2 small lesions, one in the left intermediate occipital region (Fig. 4A) and another in the right cerebellar hemisphere. These findings were consistent with a subacute stroke. The posterior extent of the lesion was located 16 mm from the occipital pole, from which the lesion extended 21 mm anteriorly and spared 11 mm of the most anterior aspect of the left cal-carine fissure. Transesophageal echocardiography revealed a large persistent foramen ovale (PFO) with atrial septum aneurysm. Due to the cerebral embolic stroke, an inter-ventional PFO closure was planned. Lesion Analysis Using similar methodology as in Case 1, we found that the lesion causing the visual field defect was located in the upper rostral portion of the primary visual cortex (Slice 12, Fig. 4C), sparing a small anterior part of the visual cortex as well as the occipital pole. According to the cytoarchitectonic maps, the probability was very low that the optic radiation was also affected (Fig. 4B). DISCUSSION HVFDs typically involve the central 10 of vision. To the best of our knowledge, excluding reports of damage to the monocular temporal crescent, there are only 3 documented cases of homonymous scotomas beyond 25 from fixation (4-6). Various reasons may account for the paucity of such reports. First, peripheral HVFDs may remain unnoticed by Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 7 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 2. Neuroimaging findings and stereotaxic probabilistic cytoarchitectonic maps for Case 1. (A) Axial FLAIR MRI 12 months after the cerebral infarct. There is a low-density area in the right occipital cortex that spares the occipital pole and the most anterior part of the visual cortex. Signs of cortical atrophy of the right occipital pole are also observed. (B) Plot of the lesion borders on the stereotaxic probabilistic cytoarchitectonic maps of the optic radiation and the primary visual cortex (C). The color bar indicates the absolute frequency of voxels containing the optic radiation and the primary visual cortex from 1 (dark blue) individual brain to 10 (red, overlap of all 10 brains). The superimposed pink contour represents the lesion borders. The highest relative frequency is observed in Slices 12 and 16 and indicates involvement of both the optic radiation (B) and the intermediate visual cortex (C). 8 Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 3. Case 2. (A) 30 threshold-related, slightly supraliminal automated static perimetry. There are a few defects (red circles) on the right outer border in both eyes. (B) Threshold-related, slightly supraliminal automated static perimetry of the 90 visual field. Right homonymous absolute peripheral scotomas, extending from 30 to 60 , are present in both eyes. Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 9 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 4. Neuroimaging findings and stereotaxic probabilistic cytoarchitectonic maps for Case 2. (A) Axial FLAIR MRI shows a lesion in the left occipital cortex with sparing of the occipital pole. (B) Plot of the lesion borders on the probabilistic stereotaxic cytoarchitectonic maps of the optic radiation and the primary visual cortex (C). The color bar indicates the absolute frequency of voxels containing the optic radiation and the primary visual cortex from 1 (dark blue) individual brain to 10 (red, overlap of all 10 brains). The superimposed pink contour represents the lesion border of the patient. The highest relative frequency is observed in Slices 12 and 16 and supports involvement of the intermediate visual cortex (C). 10 Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. the patient because of low spatial resolution of the pe-ripheral visual field. Second, the rarity of pure peripheral HVFDs may be due to anatomical variations leading to collateral circulation in cases of calcarine artery occlusion (19-22). Third, when a retrochiasmal lesion is suspected, automated static perimetry is usually performed within the central 30 of fixation. In both of our cases, the presence of single-defect points on the outer border of the 30 visual field in both eyes aroused suspicion of a homonymous pattern and led to examination of the peripheral visual field. Such subtle defects may sometimes escape detection or be erroneously interpreted as perimetric artifacts. With semi-automated kinetic perimetry in Case 1 and supraliminal automated static perimetry within 90 of fixation in Case 2, the peripheral homonymous defects were accurately detected. As to the most appropriate perimetric technique for detecting occipital pole lesions, Wong and Sharpe (23) found that manual kinetic perimetry, either with tangent screen or Goldmann technique, coupled with automated static perimetry (Humphrey Field Analyzer, central 30-2 threshold program) are satisfactory screening tests. Our results confirm this observation. Three retinotopic maps based on individuals with oc-cipital lobe lesions have been proposed regarding visual field representation in the striate cortex. According to Holmes map, approximately 25% of the surface area of the striate cortex is devoted to the central 15 of vision (1,24,25). This has been corroborated with CT (26-28) and positron emission tomography (29). Horton and Hoyt (2) advocated a revised map based on MRI data of 3 patients with occipital lobe lesions, in which the central 15 of vision are repre-sented by approximately 70% of the total surface area of the human striate cortex. Wong and Sharpe (3) challenged this finding by reviewing MRI data from 14 patients with oc-cipital lobe lesions and concluded that the central 15 of vision occupy 37% of the total surface of the human striate cortex. To determine the location of homonymous scotomas in our patients, we correlated the MRI findings with the proposed retinotopic maps. The map of Horton and Hoyt predicted that the scotomas would begin around 10 from fixation, the refined map of Wong and Sharpe predicted that the scotomas would be located at 15 , and Holmes map predicted that the scotomas would be located at 25 . Our data are consistent with the Holmes map, and support the hypothesis of Mejico et al (4), suggesting that the Horton- Hoyt and the Wong-Sharpe maps may overestimate the area of the striate cortex devoted to the central visual field. In order to exactly localize the brain lesion onto the visual pathway (optic radiation and primary visual cortex) and to investigate its relation to the functional (perimetric) out-come, we used a lesion analysis that combined established reconstruction techniques (17) with the stereotaxic proba-bilistic cytoarchitectonic atlas developed by the Ju¨lich group (13-16). This methodical approach has been pre-viously used in studies investigating the anatomy of the pupillary light reflex pathway (30), the functional topog-raphy of early periventricular lesions in regard to cerebral palsy and reorganization of language (31), the topography of unilateral tactile agnosia (32), and the involvement of damaged white matter fiber tracts in acute spatial neglect (33). Cytoarchitectonic maps are now available for a variety of brain areas, including primary motor, somatosensory, and visual cortices (34-36). In contrast to plotting the lesion onto the reference brain of the Talairach and Tournoux atlas (37), or the Montreal Neurological Institute (MNI) single-subject or group templates (38), these prob-abilistic cytoarchitectonic maps are based on the analysis of the cytoarchitecture of a sample of 10 human postmortem brains (http://www.fz-juelich.de/ime/ime_brain_mapping) and provide stereotaxic information on the location and variability of cortical areas in the MNI reference space. The technique used in our study overcomes the considerable intersubject variability of anatomical landmarks that occurs in the striate cortex (39). The prognosis and recovery of function with visual field defects depends on the localization of occipital lobe infarctions. Celebisoy et al (40) found that striate cortex involvement was associated with poor prognosis. Similarly, Messing and Ganshirt (41) suggested that the best recovery was recorded after lesions of the occipital pole, while those in the striate area had the poorest prognosis. However, caution is needed when correlating the anatomical imaging data with the functional perimetric findings because the areas of infarct may be surrounded by edema especially in the acute phase. This might confound the delineation of the actual lesion area, where irreversible neuronal death has occurred (3,7). The cytoarchitectonic maps support the localization of brain lesions (optic radiation and striate cortex) but do not provide any detail of retinotopic map-ping. They provide a useful adjunct to retinotopic maps and conventional MRI. ACKNOWLEDGMENT The authors are indebted to Mrs Elke Krapp and Regina Hofer for their help in preparation of this manuscript. REFERENCES 1. Holmes G. The organization of the visual cortex in man. Proc R Soc Lond B Biol Sci. 1945;132:348-361. 2. Horton JC, Hoyt WF. The representation of the visual field in human striate cortex. A revision of the classic Holmes map. Arch Ophthalmol. 1991;109:816-824. 3. Wong AM, Sharpe JA. Representation of the visual field in the human occipital cortex: a magnetic resonance imaging and perimetric correlation. Arch Ophthalmol. 1999;117: 208-217. 4. Mejico LJ, Bergloeff J, Miller NR. Peripheral homonymous scotomas from a cavernous angioma affecting fibers subserving the intermediate region of the striate cortex. Am J Ophthalmol. 2001;132:440-443. Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 11 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 5. Reche-Sainz JA, Domingo-Gordo B, Toledano-Ferna´ndez N. [Peripheral homonymous hemianopia. A case report]. Arch Soc Esp Oftalmol. 2005;80:475-478. 6. Barton JJS, Benatar M. Field of Vision. A Manual and Atlas of Perimetry. Totowa, NJ: Humana Press, 2003:303. 7. McFadzean R, Brosnahan D, Hadley D, Mutlukan E. Representation of the visual field in the occipital striate cortex. Br J Ophthalmol. 1994;78:185-190. 8. Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. 9. Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889-893. 10. DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2382-2386. 11. Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, Wandell BA. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis. 2003;3:586-598. 12. Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366-383. 13. Amunts K, Zilles K. Advances in cytoarchitectonic mapping of the human cerebral cortex. Neuroimaging Clin N Am. 2001;11:151-169. 14. Bu¨rgel U, Schormann T, Schleicher A, Zilles K. Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. Neuroimage. 1999;10:489-499. 15. Bu¨rgel U, Amunts K, Hoemke L, Gilsbach JM, Zilles K. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage. 2006;29:1092-1105. 16. Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and howvariable? Neuroimage. 2000;11:66-84. 17. Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191-200. 18. Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14:486-500. 19. Smith CG, Richardson W. The course and distribution of the arteries supplying the visual (striate) cortex. Am J Ophthalmol. 1966;61:1391-1396. 20. Marinkovic SV, Milisavljevic MM, Lolic-Draganic V, Kovacevic MS. Distribution of the occipital branches of the posterior cerebral artery. Correlation with occipital lobe infarcts. Stroke. 1987;18:728-732. 21. Kitajima M, Korogi Y, Kido T, Ikeda O, Morishita S, Takahashi M. MRI in occipital lobe infarcts: classification by involvement of the striate cortex. Neuroradiology. 1998; 40:710-715. 22. Lepore FE. The preserved temporal crescent: the clinical implications of an ‘‘endangered'' finding. Neurology. 2001; 57:1918- 1921. 23. Wong AM, Sharpe JA. A comparison of tangent screen, Goldmann, and Humphrey perimetry in the detection and localization of occipital lesions. Ophthalmology. 2000;107: 527-544. 24. Inouye T. Die Sehsto¨rungen bei Schussverletzungen der kortikalen Sehspha¨re nach Beobachtungen an Versundeten der letzten Japanische Kriege. Leipzig, Germany: W. Engelmann, 1909. 25. Holmes G, Lister WT. Disturbances of vision from cerebral lesions with special reference to the cortical representation of the macula. Brain. 1916;39:34-73. 26. McAuley DL, Russell RW. Correlation of CAT scan and visual field defects in vascular lesions of the posterior visual pathways. J Neurol Neurosurg Psychiatry. 1979;42: 298-311. 27. Spector RH, Glaser JS, David NJ, Vining DQ. Occipital lobe infarctions: perimetry and computed tomography. Neurology. 1981;31:1098-1106. 28. Kattah JC, Dennis P, Kolsky MP, Schellinger D, Cohan SL. Computed tomography in patients with homonymous visual field defects-clinico-radiologic correlation. Comput Tomogr. 1981;5:301-312. 29. Fox PT, Miezin FM, Allman JM, Van Essen DC, Raichle ME. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987;7: 913-922. 30. Papageorgiou E, Ticini LF, Hardiess G, Schaeffel F, Wiethoelter H, Mallot HA, Bahlo S, Wilhelm B, Vonthein R, Schiefer U, Karnath HO. The pupillary light reflex pathway: cytoarchitectonic probabilistic maps in hemianopic patients. Neurology. 2008;70:956-963. 31. Staudt M, Ticini LF, Grodd W, Kra¨geloh-Mann I, Karnath HO. Functional topography of early periventricular brain lesions in relation to cytoarchitectonic probabilistic maps. Brain Lang. 2008;106:177-183. 32. Ho¨mke L, Amunts K, Bo¨nig L, Fretz C, Binkofski F, Zilles K, Weder B. Analysis of lesions in patients with unilateral tactile agnosia using cytoarchitectonic probabilistic maps. Hum Brain Mapp. 2009;30:1444-1456. 33. Karnath HO, Rorden C, Ticini LF. Damage to white matter fiber tracts in acute spatial neglect. Cereb Cortex. 2009; 19:2331-2337. 34. Eickhoff S, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325-1335. 35. Eickhoff S, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage. 2006;32: 570-582. 36. Eickhoff S, Paus T, Caspers S, Grosbras MH, Evans A, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511-521. 37. Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart, Germany: Thieme, 1988. 38. Evans AC, Marrett S, Neelin P, Collins L, Worsley K, Dai W, Milot S, Meyer E, Bub D. Anatomical mapping of functional activation in stereotactic coordinate space. Neuroimage. 1992;1:43-53. 39. Stensaas SS, Eddington DK, Dobelle WH. The topography and variability of the primary visual cortex in man. J Neurosurg. 1974;40:747-755. 40. Celebisoy M, Celebisoy N, Bayam E, Ko¨se T. Recovery of visual-field defects after occipital lobe infarction: a perimetric study. J Neurol Neurosurg Psychiatry. 2011; 82:695-702. 41. Messing B, Ganshirt H. Follow-up of visual field defects with vascular damage of the geniculostriate visual pathway. Neuroophthalmology. 1987;4:231-242. 12 Papageorgiou et al: J Neuro-Ophthalmol 2012; 32: 5-12 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |