| Title |
New Concepts in the Diagnosis and Management of Optic Nerve Sheath Meningioma |
| Creator |
N. R. Miller |
| Affiliation |
Wilmer Eye Institute, Baltimore, Maryland, USA |
| Abstract |
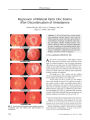
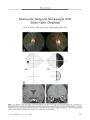
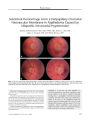
Optic nerve sheath meningiomas are by far the most common tumors of the optic nerve sheath. The diagnosis can be suspected in most cases from clinical findings and supported by the results of neuroimaging, obviating tissue biopsy in the majority of cases. Observation may be appropriate in patients with mild or no visual deficit or in whom visual loss is not progressing, whereas stereotactic fractionated radiation therapy has been demonstrated to improve or stabilize vision in progressive or advanced cases. Attempts at surgical excision, and even biopsy, of optic nerve sheath meningiomas are associated with a high risk of blindness and should be reserved for the rare case of an anteriorly located, primarily exophytic tumor with focal involvement of the dural sheath. |
| Subject |
Humans; Meningeal Neoplasms, diagnosis; Meningeal Neoplasms, therapy; Meningioma, diagnosis; Meningioma, therapy; Optic Nerve Neoplasms, diagnosis; Optic Nerve Neoplasms, therapy |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225531 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6s217mp/225531 |