| Title |
Orbital Apex Syndrome From Gnathostomiasis |
| Creator |
Preechawat, P; Wongwatthana, P; Poonyathalang, A; Chusattayanond, A |
| Affiliation |
Department of Ophthalmology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. |
| Abstract |
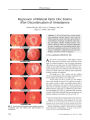
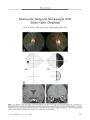
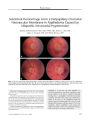
A 16-year-old Thai girl presented with acute unilateral visual loss, proptosis, and ophthalmoparesis. CT demonstrated thickening and enhancement of orbital tissues including the orbital apex. A history of consumption of raw fish, together with the findings of cutaneous migratory swelling and eosinophilia, made the diagnosis of gnathostomiasis likely. Her serum was positive for Gnathostoma spinigerum using an immunoblotting technique. Parasites removed from the skin lesions revealed the typical head bulbs with 4 circumferential rows of hooklets and fine cuticular spines on their surface. Treatment with an antihelminthic and systemic corticosteroids led to resolution of orbital inflammation but left a persistent optic neuropathy marked by nerve fiber bundle visual field loss with normal visual acuity. Gnathostomiasis should be suspected in patients with an orbital apex syndrome who live or travel in an endemic area, have eaten raw fish, and develop a migratory skin rash. |
| Subject |
Adolescent; Animals; Eye Infections, Parasitic, etiology; Female; Gnathostoma; Humans; Orbital Diseases, etiology; Spirurida Infections, complications; Tomography, X-Ray, methods; Vision Disorders, etiology |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225525 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6s217mp/225525 |