| Title |
Pipeline Embolization Device: A New Source for Embolic Retinal Vascular Occlusion |
| Creator |
Sise, Adam B; Osher, James M; Kolsky, Martin P; Stemer, Andrew; Bank, William O; Garfinkel, Richard A |
| Affiliation |
Department of Ophthalmology (ABS), Georgetown University Hospital, Washington, District of Columbia; Medstar Washington Hospital Center (ABS, JMO, MPK), Washington, District of Columbia; The Retina Group of Washington (JMO, RAG), Chevy Chase, Maryland; Cincinnati Eye Institute (JMO), Cincinnati, Ohio; and Neurointerventional Service (AS, WOB), Medstar Washington Hospital Center, Washington, District of Columbia |
| Abstract |
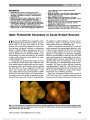
A 57-year-old woman underwent treatment of a left internal carotid artery aneurysm with a Pipeline embolization device. She subsequently experienced multiple branch retinal artery occlusions in her left eye. Although rare, ophthalmic complications may follow this new technique in the treatment of intracranial aneurysms. |
| Subject |
Embolization, Therapeutic; Female; Humans; Imaging, Three-Dimensional; Magnetic Resonance Angiography Middle Older people; Retinal Artery Occlusion; Retinal Diseases |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227516 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6c282jn/227516 |