Contents | 9 of 33
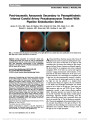
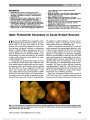
Post-traumatic Amaurosis Secondary to Paraophthalmic Internal Carotid Artery Pseudoaneurysm Treated With Pipeline Embolization Device
| Title | Journal of Neuro-Ophthalmology, December 2013, Volume 33, Issue 4 |
| Date | 2013-12 |
| Language | eng |
| Format | application/pdf |
| Type | Text |
| Publication Type | Journal Article |
| Collection | Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher | Lippincott, Williams & Wilkins |
| Holding Institution | Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management | © North American Neuro-Ophthalmology Society |
| ARK | ark:/87278/s6c282jn |
| Setname | ehsl_novel_jno |
| ID | 227537 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s6c282jn |
Page Metadata
| Title | Post-traumatic Amaurosis Secondary to Paraophthalmic Internal Carotid Artery Pseudoaneurysm Treated With Pipeline Embolization Device |
| Creator | Kim, James D; Barber, Sean M; Diaz, Orlando M; Li, Helen K; Jackson, Robert E; Hall, Drew; Lee, Andrew G |
| Affiliation | Department of Ophthalmology and Visual Sciences (JDK), University of Texas Medical Branch, Galveston, Texas; Departments of Neurological Surgery (SMB) and Interventional Neuroradiology (OMD), Methodist Neurological Institute, Houston, Texas; Department of Ophthalmology (HKL, AGL), Weill Cornell Medical College, New York, New York; School of Bioinformatics (HKL), The University of Texas Health Science Center, Houston, Texas; Department of Ophthalmology (HKL), Thomas Jefferson University, Philadelphia, Pennsylvania; Department of Ophthalmology (HKL, AGL), The Methodist Hospital, Houston, Texas; Department of Medicine (REJ, DH), The Methodist Hospital Research Institute, Houston, Texas; Department of Medicine (REJ), Weill Cornell Medical College, New York, New York; Department of Ophthalmology (AGL), Baylor College of Medicine, Houston, Texas; Departments of Ophthalmology, Neurology, and Neurosurgery (AGL), Weill Cornell Medical College, New York, New York; and Department of Ophthalmology (AGL), The University of Iowa Hospitals and Clinics, Iowa City, Iowa |
| Abstract | During evaluation for monocular visual loss, a 48-year-old woman was found to have a posttraumatic paraophthalmic internal carotid artery (ICA) pseudoaneurysm. She underwent reconstruction of the ophthalmic segment of the right ICA with a Pipeline embolization device but her vision did not return. |
| Subject | Angiography, Digital Subtraction; Blindness; Brain; Carotid Artery Injuries; Embolization, Therapeutic; Female; Fluorescein Angiography; Humans; Middle Older people; Retina; Retinal Vessels |
| Format | application/pdf |
| Publication Type | Journal Article |
| Collection | Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher | Lippincott, Williams & Wilkins |
| Holding Institution | Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management | © North American Neuro-Ophthalmology Society |
| Setname | ehsl_novel_jno |
| ID | 227512 |
| Reference URL | https://collections.lib.utah.edu/ark:/87278/s6c282jn/227512 |