| OCR Text |
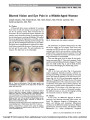
Show Mitochondrial Pseudomyasthenia Jason J. S. Barton, MD, PhD, FRCPC, John Maguire, MB, BCh, BAO, FRCPC, Michelle Mezei, MD, FRCPC, Trevor Hurwitz, MD, FRCPC, Hannah R. Briemberg, MD, FRCPC Abstract: The classic ocular motor presentation of mito-chondrial disorders is chronic, symmetric, and diffuse weakness. We describe a man with 25 years of asym-metric ptosis, ophthalmoparesis, and facial weakness that partially responded to steroid therapy. Serologic and electrophysiological investigations for myasthenia gravis were negative, but muscle biopsy confirmed a mitochon-drial myopathy. This case illustrates the potential of mi-tochondrial ophthalmoparesis to mimic the features of ocular myasthenia. Journal of Neuro-Ophthalmology 2010;30:248-251 doi: 10.1097/WNO.0b013e3181e014c8 2010 by North American Neuro-Ophthalmology Society Aclassic ocular motor presentation of mitochondrial myopathy is a chronic progressive external oph-thalmoplegia (CPEO), a slowly worsening weakness of all extraocular muscles, eyelids, and the orbicularis oculi. While asymmetric ptosis in CPEO is not uncommon, most patients do not experience diplopia because dysfunction of the extraocular muscles is symmetric (1). Markedly asym-metric ophthalmoparesis is rare (2), and when present may suggest an alternative diagnosis of ocular myasthenia gravis. We describe a man with long-standing asymmetric ptosis and ophthalmoparesis in whom ocular myasthenia was initially considered, whose signs partially responded to prednisone, but whose investigations revealed a mitochon-drial myopathy. CASE REPORT A 63-year-old man developed vertical diplopia and right ptosis abruptly at age 38. The ptosis and diplopia persisted and over the next 25 years slowly worsened, with gradually increasing separation of images. He was not aware of any diurnal variation or fatigability and denied symptoms of dysarthria, dysphagia, and limb weakness. He had complex partial seizures that began at age 25. These typi-cally began with left facial tingling, followed by tinnitus, visual hallucinations, and senses of de´ja` vu and de-realization. Electroencephalogram demonstrated sharp waves in the right central temporal region. The seizures ceased with the use of carbamazepine and lamotrigine. There was no family history of seizures or ocular disorders. His initial examination showed best-corrected visual acuity of 20/20 in both eyes, with no retinal abnormalities. Pupils were symmetric in light and dark. There was 4 mm of ptosis and partial limitations of abduction in the right eye and of adduction and depression in the left eye (Fig. 1). Measurements showed 18 prism-diopters (PD) of left hy-pertropia in primary position and downgaze, decreasing to 5 PD in upgaze, and a small exotropia in right gaze. There was subtle weakness of left lid closure (Fig. 2) but no other evidence of facial or extremity weakness. The patient was given a trial of pyridostigmine that had no effect and was then prescribed 20 mg prednisone daily. Four months after the initial prescription, he had 3 mm of right ptosis. Abduction of the right eye and depression of the left eye had greater range. The left hypertropia had decreased from 18 to 6 PD in primary position, and in downgaze, his left hypertropia had decreased to 5 PD. Two assays for acetylcholine receptor antibodies were negative. Single-fiber electromyography of the right and left frontalis muscles was performed on 2 separate occasions and was normal. Repetitive stimulation of the left facial nerve at 3 Hz did not show any decrement of the compound muscle action potential. A CT of his chest and MRI of the brain were unremarkable. Departments of Medicine (Neurology) (JJSB, MM, TH, HRB), Ophthalmology and Visual Sciences (JJSB), Pathology (JM), and Psychiatry (TH), University of British Columbia, Vancouver, British Columbia, Canada. J. J. S. Barton was supported by a Canada Research Chair and a senior scholar award from the Michael Smith Foundation for Health Research. Address correspondence to Jason J. S. Barton, MD, PhD, FRCPC, Neuro-ophthalmology Section K, VGH Eye Care Centre, 2550 Wil-low Street, Vancouver, British Columbia, Canada V5Z 3N9; E-mail: jasonbarton@shaw.ca. 248 Barton et al: J Neuro-Ophthalmol 2010; 30: 248-251 Original Contribution Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. One month later, prednisone was stopped because of confusion and depression. Four months after stopping steroid therapy, examination showed 4 mm of right ptosis; diminished abduction of the right eye; and limited ad-duction, abduction, and depression of the left eye. The left hypertropia now measured 20 PD in primary position. A biopsy of the left vastus lateralis was performed. Ragged red fibers were noted with modified Gomori tri-chrome stain, and ragged blue fibers in the nicotinamide adenine dinucleotide hydrogen (NADH) and succinic acid dehydrogenase (SDH) stains. Many cytochrome oxidase- negative muscle fibers were identified (Fig. 3). There was no evidence of inflammation. Electron microscopy showed subsarcolemmal accumulations of mitochondria with focal subsarcolemmal splitting; mitochondria displayed consid-erable pleomorphism and most contained rectangular crystalline inclusions. Mitochondrial DNA analysis was negative for deletions or point mutations of MELAS 3243, 3271 or MERFF 8344, and nuclear DNA analysis was negative for POLG and Twinkle mutations. Free and total carnitine levels were very low, at 3 and 4 mmol/L, respectively, and he was started on carnitine supplementa-tion. Electrocardiogram was normal. On 3 examinations over the subsequent 2 years, his right ptosis varied slightly from 3 to 5 mm and he remained with a 16-20 diopter left hypertropia in primary position, with exotropia varying from 0 to 18 PD in right gaze. He had occasional complaints of right leg weakness but no clear evidence of paresis in his limbs. DISCUSSION Our patient presented with weakness of the levator palpe-brae superioris and lateral rectus muscle of the right eye and of the medial rectus, inferior rectus, and orbicularis oculi of the left eye. Prednisone improved his ptosis by history, and examination confirmed better abduction of the right eye and depression of the left eye during treatment, which dete-riorated again when prednisone was stopped. Subsequent examinations also showed variability of right ptosis and extraocular movement, notably his exotropia in right gaze. About half of the patients with CPEO have asymmetric ptosis (1,3), with a few having unilateral ptosis (1,4-6). While minor asymmetries in ophthalmoparesis can occur, reflected in a 40% incidence of disconjugacy and transient or persistent diplopia (1), marked asymmetry is highly unusual. There is one report of a woman who presented with marked weakness of the right medial rectus and mild paresis of the left lateral rectus and right inferior rectus, without ptosis, combined with a right facial palsy (2). This patient showed no decremental response on nerve con-duction studies, but myopathic features on electromyog-raphy prompted muscle biopsy, ultimately leading to the finding of a mitochondrial DNA deletion, establishing the diagnosis of CPEO (7). FIG. 1. Photographic documentation of right ptosis and decreased abduction in the right eye and decreased adduction and depression in the left eye. FIG. 2. With forced lid closure, there is mild weakness of the orbicularis oculi muscle on the left. Original Contribution Barton et al: J Neuro-Ophthalmol 2010; 30: 248-251 249 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. The patient we describe did not have myopathic features on electromyography but then neither do a third of patients with mitochondrial myopathy (1). He did not have mito-chondrial DNA deletions but not all cases of CPEO do: some can have point mutations (8,9) and sometimes with overlapping features of other mitochondrial syndromes, such as MELAS or MERRF (10-12). Our patient's complex partial seizures differed from the epilepsies of these classic mitochondrial syndromes, but mitochondrial dysfunction may be a contributing factor in some cases of temporal lobe epilepsy (13). Regardless, his muscle biopsy showed clear features of a mitochondrial myopathy, and his metabolic assays revealed a carnitine deficiency, a secondary phe-nomenon in some mitochondrial myopathies (1,14,15) but not in myasthenia. Response to prednisone is typical of inflammatory or autoimmune muscle disorders, including myasthenia gravis. However, in other disorders, such as Duchenne muscular dystrophy, prednisone can also improve muscle function (16). The effect of steroids in mitochondrial myopathies is less clear. In vitro studies have suggested possible beneficial effects of methylprednisolone on mitochondrial function (17). Anecdotal reports suggest that steroids improve weakness in some patients with mitochondrial myopathy (14,18,19) but not others (2,3,20). In addition, there are descriptions of mitochondrial damage and CPEO-like signs evolving in patients on chronic steroid therapy (21). Whether our patient truly improved on prednisone can be questioned as some of his signs fluctuated without treat-ment. However, his left hypertropia was a relatively stable deficit that did show a documented decrease during the time he was taking prednisone and deterioration after he stopped. Clinically, a steroid-responsive pattern of highly asym-metric lid with facial and ocular motor weakness with variable signs between examinations strongly suggests my-asthenia gravis. Negative results of antibody testing and FIG. 3. Muscle biopsy. A. Modified Gomori trichrome-stained section showing ragged red fibers, 3400. B. Succinic acid dehydrogenase stain showing numerous fibers with increased subsarcolemmal enzymatic staining (‘‘ragged blue'' fibers), 3100. C. Cytochrome oxidase study (COX) showing ‘‘ragged brown'' fiber with increased subsarcolemmal enzymatic staining and COX negative fibers, 3400. D. Electron photomicrograph showing pleomorphic elongated mitochondria containing intramitochondrial inclusions, 343,000. Original Contribution 250 Barton et al: J Neuro-Ophthalmol 2010; 30: 248-251 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. electrophysiology should not dissuade one from the di-agnosis, given the high rate of false-negative results when myasthenia is purely ocular (22,23). Our case illustrates the potential of mitochondrial myopathy to simulate features of myasthenia, including marked asymmetry, variability, ap-parent response to steroid therapy, and lack of myopathic signs on electromyography. REFERENCES 1. Petty RK, Harding AE, Morgan-Hughes JA. The clinical features of mitochondrial myopathy. Brain. 1986;109(pt 5): 915-938. 2. Sharma NK, Gujrati M, Kumar J, Kattah JC. Chronic asymmetric progressive external ophthalmoplegia with right facial weakness: a unique presentation of mitochondrial myopathy. J Neurol Neurosurg Psychiatry. 2002;73:95. 3. Caballero PE, Candela MS, Alvarez CI, Tejerina AA. Chronic progressive external ophthalmoplegia: a report of 6 cases and a review of the literature. Neurologist. 2007;13:33-36. 4. Rossier J, Hatt M. [Atypical manifestation of progressive external ophthalmoplegia]. Klin Monatsbl Augenheilkd. 1996;208:366-367. 5. Okulla T, Kunz WS, Klockgether T, Schroder R, Kornblum C. Diagnostic value of mitochondrial DNA mutation analysis in juvenile unilateral ptosis. Graefes Arch Clin Exp Ophthalmol. 2005;243:380-382. 6. Arpa-Gutierrez FJ, Cruz-Martinez A, Campos-Gonzalez Y, Gutierrez-Molina M, Santiago-Perez S, Perez-Conde MC, Lo´pez-Pajares MR, Mart´ın-Casarrubias MA, Rubio-Mun˜oz JC, del Hoyo P, Arpa-Ferna´ndez A, Arenas-Barbero J. [Mitochondrial respiratory chain diseases. Evaluation and variability in 52 patients]. Rev Neurol. 2005;41:449-454. 7. Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S, Nonaka I, Koga Y, Spiro AJ, Brownell AKW, Schmidt B, Schotland DL, Zupanc M, DeVivo DC, Schon EA, Rowland LP. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989;320:1293-1299. 8. Hattori Y, Goto Y, Sakuta R, Nonake I, Mizuno Y, Horai S. Point mutations in mitochondrial tRNA genes: sequence analysis of chronic progressive external ophthalmoplegia (CPEO). J Neurol Sci. 1994;125:50-55. 9. Seibel P, Lauber J, Klopstock T, Marsac C, Kadenbach B, Reichman H. Chronic progressive external ophthalmoplegia is associated with a novel mutation in the mitochondrial tRNA(Asn) gene. Biochem Biophys Res Commun. 1994; 204:482-489. 10. Petruzzella V, Moraes C, Sano M, Bonilla E, DiMauro S, Schon E. Extremely high levels of mutant mtDANs co-localize with cytochrome c oxidase-negative ragged red fibers in patients harboring a point mutation at nt 3243. Human Mol Gen. 1994;3:449-454. 11. Gupta S, Brigell M, Gujrati M, Lee J. Supranuclear eye movement dysfunction in mitochondrial myopathy with tRNALEU mutation. J Neuroophthalmol. 1995;15:20-25. 12. Verma A, Moraes C, Shebert R, Bradley W. A MERRF/PEO overlap syndrome associated with the mitochondrial DNA 3243 mutation. Neurology. 1996;46:1334-1336. 13. Kudin AP, Zsurka G, Elger CE, Kunz WS. Mitochondrial involvement in temporal lobe epilepsy. Exp Neurol. 2009; 218:326-332. 14. Hsu CC, Chuang YH, Tsai JL, Jong HJ, Shen YY, Huang HL, Chen HL, Lee HC, Pang CY, Wei YH, Chen S-S. CPEO and carnitine deficiency overlapping in MELAS syndrome. Acta Neurol Scand. 1995;92:252-255. 15. Melegh B, Seress L, Bedekovics T, Kispal G, Sumegi B, Trombitas K, Me´hes K. Muscle carnitine acetyltransferase and carnitine deficiency in a case of mitochondrial encephalomyopathy. J Inherit Metab Dis. 1999;22: 827-838. 16. Angelini C. The role of corticosteroids in muscular dystrophy: a critical appraisal. Muscle Nerve. 2007;36: 424-435. 17. Martens ME, Peterson PL, Lee CP. In vitro effects of glucocorticoid on mitochondrial energy metabolism. Biochim Biophys Acta. 1991;1058:152-160. 18. Mastaglia FL, Thompson PL, Papadimitriou JM. Mitochondrial myopathy with cardiomyopathy, lactic acidosis and response to prednisone and thiamine. Aust N Z J Med. 1980;10:660-664. 19. Peterson PL. The treatment of mitochondrial myopathies and encephalomyopathies. Biochim Biophys Acta. 1995; 1271:275-280. 20. Behbehani R, Sharfuddin K, Anim JT. Mitochondrial ophthalmoplegia with fatigable weakness and elevated acetylcholine receptor antibody. J Neuroophthalmol. 2007; 27:41-44. 21. Mitsui T, Umaki Y, Nagasawa M, Akaike M, Aki K, Azuma H, Ozaki S, Odomi M, Matsumoto T. Mitochondrial damage in patients with long-term corticosteroid therapy: development of oculoskeletal symptoms similar to mitochondrial disease. Acta Neuropathol. 2002;104: 260-266. 22. Evoli A, Tonali P, Bartoccioni F, Lo Monavo M. Ocular myasthenia: diagnosis and therapeutic problems. Acta Neurol Scand. 1988;77:31-35. 23. Sanders D, Howard J. AAEE minimonograph #25: single fiber electromyography in myasthenia gravis. Muscle Nerve. 1986;9:809-819. Original Contribution Barton et al: J Neuro-Ophthalmol 2010; 30: 248-251 251 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. |