| OCR Text |
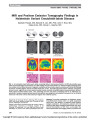
Show MRI and Positron Emission Tomography Findings in Heidenhain Variant Creutzfeldt-Jakob Disease Sashank Prasad, MD, Edward B. Lee, MD, PhD, John H. Woo, MD, Abass Alavi, MD, Steven L. Galetta, MD Abstract: The typical presentation of Heidenhain variant Creutzfeldt-Jakob disease (CJD) is a rapidly progressive visual loss in the setting of a relatively normal ophthal-mologic examination. At presentation, patients with this uniformly fatal illness frequently demonstrate only minor cortical abnormalities on MRI. Here, we document the clinical presentation and imaging results of a patient with Section Editor: Timothy J. McCulley, MD FIG. 1. A. Automated visual fields reveal rapidly worsening bilateral homonymous defects. B. MRI abnormalities were subtle and included slight hyperintensity on FLAIR imaging and restricted diffusion on diffusion-weighted imaging within the occipital cortical ribbon (arrows). A magnetic resonance perfusion study (using the unenhanced arterial spin labeling technique) revealed slightly reduced occipital blood flow (arrow). Fluorodeoxyglucose-positron emission tomography (shown in axial, sagittal, and coronal views) revealed marked occipital and parietotemporal hypometabolism (arrows). These regions included striate cortex, color processing area V4, and motion processing area V5. C. Pathologic ex-amination at autopsy revealed marked neuronal loss, gliosis, and spongiform vacuolization (arrows) within the occipital neocortex while other cortical and subcortical regions were relatively spared. Departments of Neurology (SP, SLG), Pathology (EBL), Radiology (JHW), and Nuclear Medicine Hospital (AA), University of Penn-sylvania, Philadelphia, Pennsylvania. Address correspondence to Sashank Prasad, MD, Division of Neuro-ophthalmology, Department of Neurology, Hospital of the University of Pennsylvania, 3W Gates Bldg, 3400 Spruce Street, Philadelphia, PA 19104; E-mail: sashank.prasad@uphs.upenn.edu 260 Prasad et al: J Neuro-Ophthalmol 2010; 30: 260-262 Photo Essay Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. Heidenhain variant CJD in whom abnormalities on positron emission tomographic imaging were more evident than changes on MRI. These changes were present in striate cortex and visual association areas, providing clinical-anatomical correlation with our patient's visual deficits. Nuclear imaging provides a considerably more sensitive measure of neural dysfunction early in the course of this disease. Journal of Neuro-Ophthalmology 2010;30:260-262 doi: 10.1097/WNO.0b013e3181e2aef7 2010 by North American Neuro-Ophthalmology Society A66-year-old woman noticed slowly progressive blurred vision in the left inferior visual field. There were no headaches or other accompanying symptoms. An ophthal-mologic examination revealed a homonymous left inferior field cut and no other abnormalities. Brain MRI was normal. Over several weeks, visual loss gradually extended into the right inferior visual field (Fig. 1). She was referred for neuro-ophthalmic consultation. The patient described vi-sual hallucinations in the form of shimmering orange lights in her peripheral vision along with palinopsia. Knitting had become difficult because of impaired depth perception. She had developed mild gait unsteadiness. There were no deficits of memory, language, or behavior, nor was there weakness or numbness. On examination, she was alert and fully oriented. She named 28 of 30 items on the Boston naming task and comprehended complex verbal commands. She was fluent, and she could repeat normally. On memory testing the patient recalled 10 of 10 elements in a story after a 5-minute delay. Visuospatial testing revealed difficulty copying a cube, although she drew a clock face correctly. On testing of executive functions, she named 18 words beginning with the letter F in 1 minute. She completed oral trials suc-cessfully, performed simple calculations, and demonstrated normal praxis. Corrected visual acuity was 20/25 in each eye. The patient correctly named colors but reported desaturation of blue and yellow. She identified the control Ishihara color plate but none of the test plates and made numerous errors arranging the desaturated L'anthony D-15 color panel. She was unable to perceive a stereoscopic image with the Titmus stereotest (3,000 arcsec retinal disparity). Confrontation visual fields demonstrated a dense left inferior quadrant scotoma and a partial right inferior quadrant scotoma. Within the blind field, however, she correctly discriminated motion cues (the Riddoch phenomenon). The patient reported persistence of visual images a few moments after shifting gaze but correctly described all elements of both the ‘‘cookie-thief'' picture and a Navon figure. Pupillary responses, ocular motility, and fundus examinations were normal. Strength was normal. There was no myoclonus, numbness, or dysmetria. She reached for objects accurately, without past-pointing or tremor. Tandem gait was mildly impaired. Reflexes were normal, and symmetric and plantar responses were flexor. A repeat brain MRI revealed slight abnormalities of the occipital cortical ribbon, including hyperintensity on FLAIR imaging and diffusion-weighted imaging that was more prominent on the right (Fig. 1). In addition, there were nonspecific white matter hyperintensities, consistent with small vessel ischemia. The basal ganglia and thalami were normal. An MRI perfusion study (using the unenhanced arterial spin labeling technique) revealed slightly decreased occipital blood flow. Fluorodeoxyglucose-positron emission tomography, in contrast to the MRI studies, revealed striking abnormalities, with severe hypometabolism of the bilateral occipital and parieto-temporal cortices (right greater than left) (Fig. 1). Cerebrospinal fluid analysis showed no cells, protein 52 mg/dL, glucose 56 mg/dL, and normal cytology. The 14-3- 3 immunoassay revealed only weak immunoreactivity and was considered an ambiguous result. CT of the chest and abdomen were normal. Testing for paraneoplastic anti-bodies was negative. An electroencephalogram (EEG) revealed a normal posterior dominant rhythm, without focal slowing or paroxysmal sharp waves. Visual evoked responses were normal (P100 latency, 103 milliseconds in the right eye, 101 milliseconds in the left eye). The patient went completely blind over a period of 8 weeks. She died 12 weeks from the onset of symptoms and terminally she had myoclonus and impaired arousal and orientation. At autopsy, there was severe neuronal loss and gliosis with spongiform vacuolization that predominantly affected the occipital lobes (Fig. 1). Western blot analysis demonstrated accumulation of proteinase-resistant PrPsc (type 1), and genetic sequencing revealed the homozygous methinonine polymorphism at codon 129 of the PrP gene. The Heidenhain variant of sporadic Creutzfeldt-Jakob disease (CJD) describes a rare rapidly progressing dementia in which prominent visual changes constitute the initial symptoms. In 1929, Heidenhain first described this entity, reporting 3 cases sharing this striking clinical presentation in whom histopathological analysis revealed severe abnor-malities including neuronal loss, gliosis, and vacuolization that were most prominent in the occipital lobes (1). Pub-lication of cases with similar clinical and pathological fea-tures led to the proposal that this entity be named the Heidenhain variant (2). After several decades without insight into the pathogenesis of these disorders, Stanley Prusiner (3) advanced the prion hypothesis, which implicates the misfolding of the normal PrP protein into the protease-resistant PrPsc isoform. Almost all cases of Heidenhein variant CJD (including our patient) are homozygous for methionine at codon 129 of the PrP gene, but the significance of this association remains unclear (4). The clinical diagnostic features of Heidenhain variant CJD have been well characterized (5-15). In comparison to Photo Essay Prasad et al: J Neuro-Ophthalmol 2010; 30: 260-262 261 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. patients with ataxia-predominant CJD, Heidenhain patients have a similar age at onset, although they have a more rapid deterioration (mean disease duration, 5.7 vs 7.5 months) (7). Heidenhain patients commonly report a variety of visual symptoms, including blurring, field constriction, metamorphopsia, visual hallucinations, or visual neglect. In our patient, widespread posterior meta-bolic abnormalities in striate cortex and visual association areas (including color processing area V4 and motion processing area V5) accounted for the patient's bilateral homonymous visual field defects, impaired color process-ing, and palinopsia. It is common for the brain MRI in Heidenhain variant CJD to be normal or show only minimal changes, particularly early in the disease course (8). As our case demonstrates, severe progressive cortical visual loss may occur with only minimal structural changes identified by MRI. On the other hand, several recent reports of Heidenhain variant CJD have demonstrated that nuclear imaging studies may reveal conspicuously abnormal areas of hypometabolism (9-12). Reduced occipital blood flow has also been reported using nuclear imaging techniques, including Xe-133 SPECT (5), 99mTc-SPECT (13), and [15O]H2O PET (11). In many of these cases, however, Heidenhain variant CJD was suspected without patho-logical confirmation (9-12). The case illustrated here demonstrates pathologically confirmed Heidenhain variant CJD with prominent focal hypometabolism observed on brain PET scan. In contrast, other ancillary tests (including standard MRI sequences, magnetic resonance perfusion, CSF 14-3-3, and EEG) demonstrated only mild abnormalities during the disease course and provided only limited clinical-anatomical cor-relation with our patient's visual complaints. Nuclear im-aging is a particularly sensitive indicator of the extent of neural dysfunction early in the course of Heidenhain variant CJD, demonstrating severe posterior hypometabolism in cortical regions that correlate with the visually predominant clinical deficits in these patients. REFERENCES 1. Heidenhain A. Klinische und anatomische Untersuchungen uber eine eigenartige organische Erkrankung des Zentralnervensystems im Praesenium. Z Ges Neurol Psychiatr. 1929;118:49-114. 2. Meyer A, Leigh D, Bagg CE. A rare presenile dementia associated with cortical blindness (Heidenhain's syndrome). J Neurol Neurosurg Psychiatry. 1954;17: 129-133. 3. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136-144. 4. Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, Farlow M, Dickson DW, Sima AA, Trojanowski JQ, Petersen RB, Gambetti P. Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 1996;39: 767-778. 5. Mathews D, Unwin DH. Quantitative cerebral blood flow imaging in a patient with the Heidenhain variant of Creutzfeldt-Jakob disease. Clin Nucl Med. 2001;26: 770-773. 6. Cooper SA, Murray KL, Heath CA, Will RG, Knight RS. Isolated visual symptoms at onset in sporadic Creutzfeldt- Jakob disease: the clinical phenotype of the ‘‘Heidenhain variant.'' Br J Ophthalmol. 2005;89:1341-1342. 7. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, Riedemann C, Windl O, Steinhoff BJ, Zerr I, Kretzschmar HA, Poser S. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56:55-61. 8. Jacobs DA, Lesser RL, Mourelatos Z, Galetta SL, Balcer LJ. The Heidenhain variant of Creutzfeldt-Jakob disease: clinical, pathologic, and neuroimaging findings. J Neuroophthalmol. 2001;21:99-102. 9. Clarencxon F, Gutman F, Giannesini C, Pe´nicaud A, Galanaud D, Kerrou K, Marro B, Talbot JN. MRI and FDG PET/CT findings in a case of probable Heidenhain variant Creutzfeldt-Jakob disease. J Neuroradiol. 2008;35: 240-243. 10. Grunwald F, Pohl C, Bender H, Hartmann A, Menzel C, Ruhlmann J, Keller E, Biersack HJ. 18F-fluorodeoxyglucose- PET and 99mTc-bicisate-SPECT in Creutzfeldt-Jakob disease. Ann Nucl Med. 1996;10:131-134. 11. Thomas A, Klein JC, Galldiks N, Hilker R, GrondM, Jacobs AH. Multitracer PET imaging in Heidenhain variant of Creutzfeldt- Jakob disease. J Neurol. 2006;253:258-260. 12. Tsuji Y, Kanamori H, Murakami G, Yokode M, Mezaki T, Doh-ura K, Taniguchi K, Matsubayashi K, Fukuyama H, Kita T, Tanaka M. Heidenhain variant of Creutzfeldt-Jakob disease: diffusion-weighted MRI and PET characteristics. J Neuroimaging. 2004;14:63-66. 13. Pichler R, Ciovica I, Rachinger J, Weiss S, Aichner FT. Multitracer study in Heidenhain variant of Creutzfeldt-Jakob disease: mismatch pattern of cerebral hypometabolism and perfusion imaging. Neuro Endocrinol Lett. 2008;29: 67-68. 14. Hopf HC, Althaus HH, Sabuncu S. [Type Heidenhain of the subacute spongious encephalopathy (SSE) Creutzfeldt-Jakob (author's transl)]. Z Neurol. 1974;206: 149-156. 15. Bosch PL, Vass K. [Subacute spongious encephalopathy of the Heidenhain type]. Nervenarzt. 1975;46:160-162. Photo Essay 262 Prasad et al: J Neuro-Ophthalmol 2010; 30: 260-262 Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. |