| OCR Text |
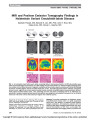
Show Advanced Imaging of Anterior Visual Pathway Ischemia: State of the Art and Future Directions Edward P. Quigley III, MD, PhD, Anne Osborn, MD It is truly an exciting time to be neuroophthalmologists and neuroradiologists. Our two subspecialties are accelerating together. As magnetic resonance imaging (MRI) improves, we neuroradiologists can provide confirmation and evaluation of pathologies previously identified only clinically. Two articles appearing in this issue of the Journal of Neuro-Ophthalmology (1,2) demonstrate this coalescence of clinical acumen and improvements in neuroimaging. In particular, recent advances in MRI of acute ischemia facilitate the brave new world of visual pathway imaging. Higher field magnets, improved coil arrays, and new MR sequences are all contributing factors. In particular, diffusion-weighted imaging (DWI) has been improved upon with diffusion tensor imaging (DTI). In this editorial, we will discuss the imaging advances in the context of the two reports appearing in the Journal. Next, we would like to introduce current and some future imaging advances on the near horizon. In addition, we will discuss the hurdles and limitations of imaging on the cutting edge of ischemic optic pathology. ‘‘MRI restricted diffusion in optic nerve infarction after autologous fat transplantation,'' by Lee et al (1) is an elegant display of imaging complementing the clinical evaluation. In the patient described with acute post procedural deficits from autologous fat transplantation, ischemia in the middle cerebral artery distribution did not explain the clinical deficit. Careful analysis of routinely acquired MR imaging demonstrated additional pathology along the optic nerve. As in brain parenchyma, if diffusion hy-perintensity is present, then one should evaluate the corresponding apparent diffusion coefficient (ADC) map values. In this case, ADC values were both qualitatively and quantitatively decreased. This excludes T2 shine through phenomenon. The ADC values obtained are comparable to brain paren-chymal ischemic values. This paper demonstrates the critical role of considering external carotid to internal carotid arterial anastamoses. Retrograde injury to the optic nerve has demonstrated via em-bolization to the internal carotid and ophthalmic arteries (3). ‘‘Chiasmal stroke following open heart surgery,'' by Fabian et al (2) provides a superb example of DWI detection of small regions of ischemia. While the optic chiasm is vascularly rich, it is still susceptible to ischemic or compressive injury. Pathology of the sella can compress or remodel the chiasm and embolic phenomenon can cause acute ischemia. While subtle increased diffusion restriction is seen in the chiasm in the case reported by Fabian and colleagues, the decrease in ADC value is compelling. Like routine parenchymal ischemia, DWI is the first MR sequence to become positive for ischemia, then FLAIR, followed by T2. This paper demonstrates the evolution of ischemic chiasmatic T2 hyper-intensity four days post ischemia with thin section orbital MRI. These papers introduce some of key concepts in the modern imaging of the visual pathways (4) and we would like to make some additional recommendations. Routine DWI may depict larger lesions of the optic nerve chiasm or optic radiations. DTI uses multiple diffusion directions of analysis rather than three direction conventional DWI. If possible, both DWI and DTI should be performed at 3 mm intervals rather than routine whole brain 5 mm intervals. Even comparing thin section DWI to DTI, diffusion tensor imaging may better detect small lesions due to higher signal to noise ratio. Imaging limitations are always time and motion degradation. If the patient cannot maintain position for 5-6 minutes for DTI acquisition, routine DWI may be the only imaging choice available. Neuroradiology Section, Department of Radiology, University of Utah School of Medicine, Salt Lake City, Utah. Address correspondence to Edward P. Quigley III, MD, PhD, Neuroradiology Section, Department of Radiology, University of Utah School of Medicine, Salt Lake City, UT; E-mail: edward.quigley@hsc.utah.edu Quigley and Osborn: J Neuro-Ophthalmol 2010; 30: 213-215 213 Editorial Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. Ocular motion can significantly degrade both DWI and DTI of the retrobulbar optic nerve. However, DTI is superb for detection of small ischemic lesions. For example, infarcts of the small structures such as the fornices, septum pellu-cidum, chiasm, hippocampus, and medial longitudinal fasciculus can be seen on DTI (5-7). Consequently, if ischemic optic neuropathy is suspected clinically, then obtain DWI, DTI, thin section coronal STIR and T2 fat-saturated imaging. Coronal STIR and T2 demonstrate optic nerve and chiasm in cross section. ADC values can be quantitatively measured on vendor specific software or open source imaging software (8). Due to the small size of the optic nerve, volume averaging of adjacent fat, muscle or bone may significantly affect measurements (9,10). Point ADC values can be extracted or qualitative maps can be generated (Fig. 1). In contrast to normal brain parenchyma, the visual pathways are a highly organized group of fibers. Similar to analysis of DWI and DTI in the spinal cord, one can further divide optic nerve diffusion parameters into longitudinal and axial components (11,12). There are differential de-creases in diffusion parameters in optic neuritis (13,14). However, for ischemic optic neuropathy, these differences have not yet been fully evaluated. Factors that limit quantitative analysis are small size of structures, limited signal-to-noise ratio, susceptibility from orbital fat and aerated sinuses, and ‘‘wrap-around'' artifact from small field of view (15). Jeong et al have developed IMIV DTI, in-terleaved multiple inner volume diffusion tensor imaging, to address this last issue (16,17). We have applied this method to optic neuritis and hope to apply to acute optic pathway ischemia (Fig. 1). To address the low signal from optic nerves, Parker and Hadley have developed an optic nerve surface coil for 3T imaging (18). Future imaging advances in visual pathway imaging will include diffusion spectrum imaging and diffusion kurtosis imaging. Diffusion spectrum imaging can resolve crossing fiber pathways like the chiasm (19). Diffusion kurtosis addresses linear assumptions in conventional DTI (20). These methods have the potential to analyze the chiasmatic decussation. Clinical applications of higher magnetic field strength MR and improved tractography techniques will continue to elucidate anatomy and pathology (21-23). In conclusion, neuroradiology is applying new techni-ques to better image the visual pathway from the fundus to the calcarine cortex. We now can provide imaging evidence to support astute neuro-ophthalmologic examination. MRI, particularly DTI, can now demonstrate the acute onset and subsequent evolution of visual pathway ischemia. Ongoing advances in MR sequences, more powerful magnets, and new coil arrays provide complementary imaging which will continue to improve in months and years to come. REFERENCES 1. Lee YJ, Kim HJ, Choi K-D, Choi H-Y. MRI restricted diffusion in optic nerve infarction after autologous fat transplantation. J Neuroophthalmol. 2010;30:216-218. 2. Fabian ID, Greenberg G, Huna-Baron R. Chiasmal stroke following open-heart surgery. J Neuroophthalmol. 2010;30: 219-221. 3. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80:1026-1027. 4. Wippold FJ, Cornelius RS, Broderick DF, Brown DC, Brunberg JA, Davis PC, De La Paz RL, Garvin CF, Germano I, McConnell CT Jr, McDermott MW, Mukherji SK, Seidenwurm DJ, Sloan MA, Smirniotopoulos JG. Orbits, vision, and visual loss. AJNR Am J Neuroradiol. 2010;1: 196-198. 5. Adamovich BL, Gualberto G, Roberts T, HautMW, Gutmann L. Teaching neuroimages: amnesia due to fornix infarction. Neurology. 2009;73:e86. 6. Hattingen E, Rathert J, Raabe A, Anjorin A, Lanfermann H, Weidauer S. Diffusion tensor tracking of fornix infarction. J Neurol Neurosurg Psychiatry. 2007;78:655-656. 7. Shah LM, Davidson HC, Rassner U,Wiggins R,Wold J, Digre K, Warner J, Quigley EP. Novel diffusion tensor imaging detection of medial longitudinal fasciculus ischemia in patients with intranuclear ophthalmoplegia. Scientific paper presented at: ASNR, Boston; 2010. 8. Medinria. Available at: http://www-sop.inria.fr/asclepios/. Accessed July 22, 2010. FIG. 1. A. 3 Tesla axial T2 imaging of normal optic nerves B. High-resolution DTI using IMIV DTI and custom optic nerve coil array (16,18) in a patient with bilateral optic neuritis. Yellow arrow points to the more heavily affected intraorbital left optic nerve. C. Tractography performed using IMIV DTI data. There is loss of normal fiber tracking in the more affected left intraorbital optic nerve (red arrow). 214 Quigley and Osborn: J Neuro-Ophthalmol 2010; 30: 213-215 Editorial Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. 9. Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45:770-780. 10. Sarlls JE, Pierpaoli C. In vivo diffusion tensor imaging of the human optic chiasm at sub-millimeter resolution. Neuroimage. 2009;47:1244-1251. 11. Kim TH, Zollinger L, Shi XF, Rose J, Patel A, Kim SE, Jeong EK. Quantification of diffusivities of human cervical spinal cord using a 2D single shot interleaved multi-slice inner volume diffusion weighted echo planner imaging (2D ss-IMIV-DWEPI) technique. AJNR Am J Neuroradiol. 2010;31: 682-687. 12. Naismith RT, Xu J, Tutlam NT, Trinkaus K, Cross AH, Song SK. Radial diffusivity in remote optic neuritis discriminates visual outcomes. Neurology. 2010;74:1702- 1710. 13. Trip SA, Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, Plant GT, Miller DH. Optic nerve diffusion tensor imaging in optic neuritis. Neuroimage. 2006;30: 498-505. 14. Quigley EP, Anderson JS, Warner J, Hadley JR, Kim SE, Jeong EK, Parker DL, Rose J. Diffusion tensor imaging of the human optic nerve in multiple sclerosis. Scientific paper presented at: ASNR, New Orleans; 2008. 15. Xu J, Sun SW, Naismith RT, Snyder AZ, Cross AH, Song SK. Assessing optic nerve pathology with diffusion MRI: from mouse to human. NMR Biomed. 2008;21:928-940. 16. Jeong EK, Kim SE, Guo J, Kholmovski EG, Parker DL. High-resolution DTI with 2D interleaved multislice reduced FOV single-shot diffusion-weighted EPI (2D ss-rFOV-DWEPI). Magn Reson Med. 2005;54:1575-1579. 17. Jeong EK, Kim SE, Kholmovski EG, Parker DL. High-resolution DTI of a localized volume using 3D single-shot diffusion-weighted STimulated echo-planar imaging (3D ss-DWSTEPI). Magn Reson Med. 2006;56: 1173-1181. 18. Parker DL, Hadley JR. Multiple-region gradient arrays for extended field of view, increased performance, and reduced nerve stimulation in magnetic resonance imaging. Magn Reson Med. 2006;56:1251-1260. 19. Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1): S205-S223. 20. Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53: 1432-1440. 21. Roebroeck A, Galuske R, Formisano E, Chiry O, Bratzke H, Ronen I, Kim DS, Goebel R. High-resolution diffusion tensor imaging and tractography of the human optic chiasm at 9.4 T. Neuroimage. 2008;39:157-168. 22. Hofer S, Karaus A, Frahm J. Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Frontiers Neuroanatomy. 2010;4:15. 23. Nickerson JP, Salmela MB, Koski CJ, Andrews T, Filippi CG. Diffusion tensor imaging of the pediatric optic nerve: intrinsic and extrinsic pathology compared to normal controls. J Magn Reson Imaging. 2010;32:76-81. Quigley and Osborn: J Neuro-Ophthalmol 2010; 30: 213-215 215 Editorial Copyright © North American Neuro-ophthalmology Society.Unauthorized reproduction of this article is prohibited. |