| Title |
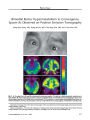
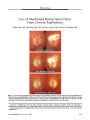
Atypical teratoid/rhabdoid tumor arising from the third cranial nerve. |
| Creator |
Wykoff, Charles C; Lam, Byron L; Brathwaite, Carole D; Biegel, Jaclyn A; McKeown, Craig A; Rosenblum, Marc K; Allewelt, Heather B; Sandberg, David I |
| Affiliation |
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine and Miami Children's Hospital, Miami, Florida 33136, USA. |
| Abstract |
An otherwise healthy 6-week-old girl who presented with an isolated left third cranial nerve palsy underwent MRI that revealed an enhancing mass intrinsic to the left third cranial nerve. Rapid enlargement of the lesion over 1 month led to subtotal neurosurgical resection of an atypical teratoid/rhabdoid tumor (AT/RT), a rare, highly aggressive malignancy of infancy closely related histologically to medulloblastoma and primitive neuroectodermal tumor. Despite aggressive chemotherapy, the patient died within 6 months of presentation. This is the first report of an AT/RT presenting as an isolated third cranial nerve palsy caused by tumor arising from within the nerve. |
| Subject |
Antineoplastic Combined Chemotherapy Protocols; Cranial Nerve Neoplasms; Disease Progression; Fatal Outcome; Female; Humans; Hydrocephalus; Infant; Magnetic Resonance Imaging; Meningeal Carcinomatosis; Mesencephalon; Neoplasm Invasiveness; Neoplasm Recurrence, Local; Neurosurgical Procedures; Oculomotor Nerve; Oculomotor Nerve Diseases; Rhabdoid Tumor; Subarachnoid Space; Teratoma; Treatment Failure; Tumor Markers, Biological |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225725 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6gj2q18/225725 |