| Title |
Upbeat nystagmus from a demyelinating lesion in the caudal pons. |
| Creator |
Tilikete, Caroline; Milea, Dan; Pierrot-Deseillgny, Charles |
| Affiliation |
Neuro-Ophthalmology Unit, Hpital Neurologique, Hospices Civils de Lyon, 59 Bd Pinel, 69 677, Bron, France. |
| Abstract |
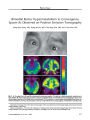
A 51-year-old man developed positional vertigo, ataxia, dysgeusia, diplopia, and oscillopsia. Eye movement examination and video-oculographic recording disclosed primary position upbeat nystagmus (PPUN) and a right internuclear ophthalmoplegia. Brain MRI showed a small focal lesion in the right dorsal tegmentum of the caudal pons with signal characteristics consistent with a primary demyelinating central nervous system disease. PPUN has not been described previously with a lesion in such a location. Clinicoanatomic correlation in this patient suggests that a lesion of the superior vestibular nucleus and its efferent crossing ventral tegmental tract could be responsible for the PPUN. This case report contributes to a better understanding of the role of this pathway in humans. |
| Subject |
Ataxia; ; Demyelinating Diseases; Disease Progression; Efferent Pathways; Humans; Magnetic Resonance Imaging; Male; Middle Older people; Nystagmus, Pathologic; Ocular Motility Disorders; Oculomotor Nerve; Pons; Reflex, Vestibulo-Ocular; Reticular Formation; Taste Disorders; Trochlear Nerve; Vertigo; Vestibular Nuclei |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225724 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6gj2q18/225724 |