| OCR Text |
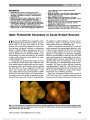
Show Bilateral Nonarteritic Anterior Ischemic Optic Neuropathy: Comparison of Visual Outcome in the Two Eyes Sohan Singh Hayreh, MD, PhD, DSc, FRCS, FRCOphth (Hon), M. Bridget Zimmerman, PhD Background: In patients with bilateral sequential nonarter-itic anterior ischemic optic neuropathy (NA-AION), previous studies have reported conflicting results on whether or not the visual outcome is similar in the 2 eyes. The authors investigated this issue in 174 consecutive patients with bilateral NA-AION. Methods: At the first visit, all patients had a detailed ophthalmic and medical history and comprehensive ophthal-mic evaluation. Visual evaluation was performed by recording Snellen visual acuity and visual fields with kinetic perimetry. The same ophthalmic evaluation was performed at each follow-up visit. The data on the difference in visual acuity, which was converted to logarithm of the minimum angle of resolution (logMAR) form, and visual field defects between the first eye and the second eye of each patient at the initial visit and at the final follow-up were analyzed. A similar subgroup analysis was performed on patients treated with systemic corticosteroids. Results: At presentation, both initial visual acuity and visual field defects were significantly better in the second eye than in the first eye (P , 0.0001). As a predictor of initial visual acuity in the second eye, the initial logMAR of the first eye only explained 7.0% (R2) of the total variation in the initial logMAR of the second eye. Intraclass correla-tion (ICC) between the paired eyes was 0.19 (95% confi-dence interval [CI], 0.04-0.33), indicating poor agreement. The absolute difference in initial visual field grade between the 2 eyes was 0.5 or greater in 78%. The weighted kappa statistic for agreement between visual field defects of the 2 eyes was 0.27 (95% CI, 0.19-0.36), indicating poor agreement. At the final fol-low- up, a difference in logMAR of at least 0.3 between the paired eyes was found in 38% of the steroid-treated group and 45% of the untreated group. For visual field grade, there was a difference of at least 0.5 in 70% of those who were treated with steroid and in 76% of those not treated. The ICC for logMAR and weighted kappa for visual field grade for the paired eyes was below 0.60 for both the groups. The findings indicated poor agreement between the 2 eyes. Conclusion: The results show that in patients with bilateral sequential NA-AION, there are large differences between the visual acuity and visual field findings of paired eyes at initial and final visit, whether or not treated with steroids. It is not possible to predict the visual acuity and visual field grade in the second eye based solely on the first eye. Journal of Neuro-Ophthalmology 2013;33:338-343 doi: 10.1097/WNO.0b013e31829b5d03 © 2013 by North American Neuro-Ophthalmology Society For patients with bilateral nonarteritic anterior ischemic optic neuropathy (NA-AION), it would be useful from a prognostic point of view to know whether the visual out-come in the 2 eyes is going to be similar or not. Conflicting results have been reported in the literature (1-9) (Table 1). Because of these contradictory results, we investigated this issue in 174 consecutive patients with bilateral sequential NA-AION. METHODS We compared visual acuity and visual field defects in the 2 eyes of patients with bilateral sequential NA-AION who were seen in our clinic from 1973 to 2000, as a part of prospective studies on various aspects of NA-AION. The data were compiled from 174 consecutive bilateral NA-AION patients who fulfilled our inclusion and exclusion criteria, and the study was approved by our institutional review board. Inclusion criteria were: 1) a history of sudden visual loss, usually Department of Ophthalmology and Visual Sciences (SSH), College of Medicine, University of Iowa, Iowa City, Iowa; and Department of Biostatistics (MBZ), College of Public Health, University of Iowa, Iowa City, Iowa. Supported by grant EY-1151 from the National Institutes of Health. The authors report no conflicts of interest. Address correspondence to Sohan Singh Hayreh, MD, PhD, DSc, FRCS, FRCOphth (Hon), Department of Ophthalmology and Visual Sciences, University Hospitals and Clinics, 200 Hawkins Drive, Iowa City, Iowa 52242-1091; E-mail: sohan-hayreh@uiowa.edu 338 Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. discovered in the morning; 2) presence of optic disc edema (ODE) at onset; 3) presence of optic disc-related visual field defects; 4) no neurological, systemic, or ocular disorder, which could be responsible for ODE and visual impairment; 5) pa-tients seen within 3 months of onset of NA-AION; and 6) only patients with follow-up of at least 6 months, this is because studies (10-12) on NA-AION have shown that visual functions become stable 6 months after the onset. Exclusion criteria were: 1) patients who had any retinal or optic nerve disorder or any other factor (e.g., cataract), which might affect vision; 2) patients with unreliable visual fields; and 3) patients with incipient NA-AION (13) who had no demonstrable visual dysfunction. Studies Performed A detailed ophthalmic and medical history was obtained at the patient's first visit to our clinic (by S.S.H.). A comprehensive ophthalmic evaluation was performed at that time (by S.S.H.). When giant cell arteritis was suspected, based on systemic symptoms, elevated erythrocyte sedimentation rate, and/or C-reactive protein or suspicion of arteritic AION, a temporal artery biopsy was performed to rule out giant cell arteritis. Follow-up Protocol for all Patients Patients were followed initially every 2-4 weeks as long as there was ODE, which lasted 7.9 weeks (range, 5.8-11.4 weeks) (14). After that, they were followed at 3 months, 6 months, and then yearly. Visual Acuity This was measured with Snellen visual acuity chart and under identical testing conditions and converted to logarithm of the minimum angle of resolution (logMAR) for statistical analysis. Visual Fields Throughout this study, we used kinetic perimetry. Auto-mated perimetry did not exist when we started the study in 1973; moreover, the changing face of automated perimetry would make such long-term studies difficult. Visual field testing was attempted in all patients with a visual acuity of hand motion or better at all visits, with a kinetic perimeter using I-2e, I-4e, and V-4e targets regularly. Evaluations of Visual Acuity and Visual Field Defects Each was evaluated separately in a masked fashion, that is, changes in visual acuity and visual fields were evaluated independent of each other, so that the severity of one did not influence the evaluation of the other. Also, in eyes that developed recurrence of NA-AION, only the data from ophthalmic examination collected up to the last follow-up visit of the first episode were used, that is, before the onset of recurrence. TABLE 1. Literature summary of comparison of visual acuity in the 2 eyes of bilateral sequential NA-AION Authors Year Study Retrospective/ Prospective Number of Patients Number (%) $0.3 LogMAR Difference Between Eyes Pearson Correlation Between Eyes (r) Authors' Conclusion VA in 2 Eyes Similar/Different Georgiadès et al (1) 1966 Retrospective 6 4 (67%)* first visit 0.26* first Different 6 (100%)* last visit 20.30* last Boghen and Glaser (2) 1975 Retrospective 14 Not reported Not reported Different Kupersmith et al (3) 1997 Retrospective 33 22 (67%) 0.28 Different WuDunn et al (4) 1997 Retrospective 31 22 (71%)* 0.29* Different Newman et al (5) 2002 Prospective 128 (80/48) 53/80†‡ (66%) 0.51‡ first Different 22/48†‡ (46%) 0.29‡ first Kurz (6) 1969 Retrospective 7 Not reported Not reported Similar (no test performed) Moro et al (7) 1989 Retrospective 26 Not reported Not reported Similar (only tested for pairwise difference, NS) Boone et al (8) 1996 Retrospective 16 7 (44%)* 0.52* Similar Mercado et al (9) 2012 Retrospective 86 Not reported 0.28 Spearman Similar *Calculated from table listing the data for visual acuity for each subject in the study. †Counts obtained from figure provided in the study for last visit. ‡Reported separately for those with 80 existing NA-AION in fellow eye and 48 with new NA-AION in fellow eye. logMAR, logarithm of the minimum angle of resolution; NA-AION, nonarteritic anterior ischemic optic neuropathy; NS, nonsignificant; VA, visual acuity. Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 339 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Visual Acuity Evaluation A change of at least 3 lines in the Snellen visual acuity chart was considered a significant change, which is equivalent to a logMAR change of at least 0.30. Visual Field Evaluation The entire visual field was graded in 4 levels-from 0 (normal) to 4 (severe loss) in steps of 0.5 (and occasionally 0.25 when the differences were subtle). The method of grading visual fields is discussed at length elsewhere (11). The findings were then condensed for descriptive purposes into minimal (grade 0.5), mild (grades .0.5-1.0), moderate (grades 1.5-2.0), marked (grades 2.5-3.0), and severe (grades 3.5-4.0) loss (10-12). As shown by our previous study (11), a change of 0.5 or more in visual field grade is significant. Statistical Methods Descriptive statistics (median, interquartile range, and percen-tages) were calculated for visual acuity (logMAR) and visual field grade for the first eye and the second eye and for the difference between the paired eyes at the initial visit and at the final follow-up. Wilcoxon signed-rank test was used to test for the difference in visual acuity and visual field grade between the first eye and the second eye of the same patient. Weighted kappa statistics was used to assess the agreement in visual field grade between the first eye and the second eye of the same patient. For assessing final visual acuity and visual field grade, patients were classified into 3 groups based on the treatment received (both eyes with steroid treatment; both eyes with no treatment; and one eye treated and the other not treated). Initial visual acuity and visual field of the first eye and the second eye, and the difference between eyes were compared among the 3 groups using the Kruskal-Wallis exact test. In addition, parametric methods for measuring agreement in logMAR were also used. This required the data to have a normal distribution, which was not seen in our logMAR data. To satisfy the normality assumption, the natural log transformation was applied to the logMAR values to normalize the data distribution. Because there were negative and 0 logMAR values (corresponding to 20/15 and 20/20, respectively), the natural log transformation was applied to the logMAR value plus a constant, that is, in (logMAR + c), with c determined as the value that resulted in the largest Shapiro-Wilk normality test statistic. Statistical analysis per-formed on the transformed logMAR included: 1) Pearson correlation between the first eye and the second eye of the same patient, 2) variance component analysis, and 3) intra-class correlation (ICC). Variance component analysis and ICCs were computed as described by Shrout and Fleiss (15). For interpreting ICC and weighted kappa, agreement .0.8 is almost perfect, .0.6-0.8 substantial, .0.4-0.6 moderate, .0.2-0.4 fair, and #0.2 poor (16). RESULTS Of the 174 patients, the eye that was first diagnosed with NA-AION was the right eye in 45%, left eye in 47%, and 8% with bilateral involvement. Initial visual acuity and visual field defect in the first eye and the second eye are presented in Table 2. Initial visual acuity at presentation showed significantly better initial visual acuity in the second eye compared with the first eye (P , 0.0001). The plot of initial logMAR between the first eye and the second eye is presented in Figure 1A. In 59% of the cases, there was at least a 0.3 logMAR difference, which is considered clinically significant. TABLE 2. Initial visual acuity and visual field in the first and second eye First Eye Second Eye Difference/Agreement Visual acuity, logMAR Second-First Median (IQR) 0.4 (0.1-1.0) 0.1 (0.0-0.4) 20.1 (20.8 to 0) Range 20.1 to 5.3 20.1 to 3.3 24.3 to 3.2 Distribution Absolute difference #0.1 (20/25 or better) 61 (35%) 91 (52%) ,0.3: 72 (41%) .0.1 to #0.3 24 (14%) 30 (17%) 0.3-1.0: 58 (33%) .0.3 to #0.5 19 (11%) 15 (9%) .1.0: 44 (25%) .0.5 to #0.6 12 (7%) 8 (5%) Pearson correlation (95% CI) .0.6 to #1.3 19 (11%) 19 (11%) 0.26 (0.12-0.40), R2 = 7% .1.3 (CF or worse) 32 (18%) 11 (6%) Intraclass correlation (95% CI) 0.19 (0.04-0.33) Visual field defect Absolute difference None-Minimal (0-0.5) 21 (12%) 51 (29%) ,0.5: 38 (22%) Mild (.0.5-1.0) 17 (10%) 20 (11%) 0.5-1.0: 79 (45%) Moderate (1.5-2.0) 52 (30%) 43 (25%) .1.0: 57 (33%) Marked (2.5-3.0) 56 (32%) 44 (25%) Weighted kappa (95% CI) Severe (3.5-4.0) 28 (16%) 16 (9%) 0.27 (0.19-0.36) IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution. 340 Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Pearson correlation between the 2 eyes was 0.26. Although this correlation was statistically significant, this level of agreement was low, with the initial logMAR of the first eye only explaining 7.0% (R2) of the total variation in the initial logMAR of the second eye, indicating that it is not adequate to predict the visual acuity in the second eye based solely on the first eye. Partitioning the variance com-ponents of initial logMAR resulted in a much greater within-patient (eyes from same patient) variance compo-nent than between-patient (eye from different patients) var-iance component resulting in a low ICC of 0.19, indicating poor agreement between the paired eyes. For initial visual field defect, the weighted kappa statistic for agreement between the 2 eyes was 0.27, with 95% lower limit of 0.19, indicating fair to poor agreement. The 174 paired cases were classified into 3 groups based on the treatment received. There were 75 patients (43%) who received steroid therapy for both eyes, 42 (24%) with neither eye treated with steroids, and 57 (33%) with one eye treated with steroids but not the other. These groups did not differ significantly in initial logMAR of the first eye (P = 0.243), second eye (P = 0.347), and in the initial logMAR difference of paired eyes (P = 0.329). There was also no significant difference among the groups in initial visual field defect severity of the first eye (P = 0.354), second eye (P = 0.384), and in the initial visual field grade difference of paired eyes (P = 0.296). Assessing agreement in final visual acuity and visual field defect in the paired eyes was performed separately for the 2 groups: 1) where both eyes were treated with steroid therapy and 2) where both eyes had no treatment. Those with only one eye treated with steroids and the other not treated were not included in the analysis of final visual acuity and visual fields. For the remaining 2 groups, only paired cases, where both eyes were followed for at least 6 months, n = 60 of the 75 with both eyes treated with steroids and n = 29 of the 42 with both eyes with no steroid treatment, were used in the assessment of agreement of final visual findings in paired eyes. Final visual acuity and visual field in the first eye and the second eye by treatment group are presented in Table 3. Com-parison of final visual acuity between the first eye and the second eye in those with steroid treatment showed significantly better visual acuity in the second eye (P = 0.030). For these patients, there were 38% with logMAR difference of 0.3 or greater between the 2 eyes. For those not treated with steroids, although the median difference in final logMAR between the first eye and the second eye was not significantly different from zero (P = 0.539), the range of the logMAR differences was wide, from 22.1 to +3.9, with 21% having .1 logMAR difference. There were 45% that had 0.3 or greater logMAR difference between the 2 eyes (Table 3). The plots of final logMAR between the first eye and the second eye are shown in Figure 1B for those with steroid treatment and in Figure 1C for those without steroids. As predictor of the second eye's final logMAR, the total var-iation in the second eye that was explained by the first eye final logMAR was 14% in steroid-treated eyes and 27% in FIG. 1. Plots showing: (A) initial logarithm of the minimum angle of resolution (logMAR) visual acuity between the first and second eye in all patients, (B) final logMAR between the first and second eye in steroid-treated patients, and (C) final logMAR between the first and second eye in nontreated patients. The line of equality (dotted line) and points between 2 solid lines indicates which pairs of eyes have logMAR difference of ,0.3. Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 341 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. those without steroid treatment. The ICC for both groups was ,0.60 with the 95% confidence interval (CI) lower limit of ,0.25 (Table 3). Comparison of the final visual field defect between the first eye and the second eye in the steroid-treated group showed less degree of visual field defect in the second eye (P = 0.004). For those not treated, although median dif-ference in final visual field grade between the first eye and the second eye was not significantly different from zero (P = 0.788), the visual field grade differed by as much as 3 points, with 21% having a difference of at least 1 (Table 3). The absolute difference in visual field grade at the final follow-up between the first eye and the second eye was at least 0.5 in 70% of those who were treated with steroids and in 76% of those with no steroids. Weighted kappa statistic was ,0.5, and 95% CI lower limit was at the level of fair to poor agreement. DISCUSSION Table 1 summarizes the findings of previous studies (1-9) of visual acuity in the first eye and the second eye of patients experiencing bilateral sequential NA-AION. In general, 0.3 logMAR is a clinically significant difference between 2 visual acuities. Using that criterion, of the 9 studies that pre-sented findings on bilateral NA-AION, 3 provided either the percentage of subjects with a difference of at least 0.3 logMAR between eyes or a correlation coefficient for visual acuity between eyes and 3 studies gave a listing of the visual acuity for each pair of eyes for which the difference and the correla-tion between eyes was calculated. By applying meta-analysis under the random-effects model (17) on the findings from these 6 studies and our study (102/174 with at least 0.3 logMAR difference and correlation of r = 0.26 between eyes), a combined estimate for each of these 2 TABLE 3. Final visual acuity and visual field in the first and second eye by treatment group With steroid treatment First Eye Second Eye Difference/Agreement Visual acuity, logMAR Second-First Median (IQR) 0.2 (0.1-0.7) 0.1 (0.0-0.4) 0.0 (20.3 to 0) Range 20.1 to 3.3 20.1 to 3.3 22.9 to 1.1 Distribution Absolute difference #0.1 (20/25 or better) 26 (43%) 31 (52%) ,0.3: 37 (62%) .0.1 to #0.3 10 (17%) 11 (18%) 0.3-1.0: 15 (25%) .0.3 to #0.5 7 (12%) 12 (20%) .1.0: 8 (13%) .0.5 to #0.6 1 (2%) 2 (3%) Pearson correlation (95% CI) .0.6 to #1.3 12 (20%) 7 (12%) 0.37 (0.13-0.57), R2 = 14% .1.3 (CF or worse) 4 (7%) 2 (3%) Intraclass correlation (95% CI) 0.46 (0.24-0.64) Visual field defect Absolute difference None-minimal (0-0.5) 11 (18%) 21 (35%) ,0.5: 18 (30%) Mild (.0.5-1.0) 6 (10%) 5 (8%) 0.5-1.0: 26 (43%) Moderate (1.5-2.0) 17 (28%) 13 (22%) .1.0: 16 (27%) Marked (2.5-3.0) 16 (27%) 18 (30%) Weighted kappa (95% CI) Severe (3.5-4.0) 10 (17%) 3 (5%) 0.32 (0.16-0.48) No treatment First Eye Second Eye Difference/Agreement Visual acuity, logMAR Second-First Median (IQR) 0.1 (0.0-0.4) 0.2 (0.0-0.5) 0.0 (20.1 to 0.1) Range 20.1 to 4.3 20.1 to 4.3 22.1 to 3.9 Distribution Absolute difference #0.1 (20/25 or better) 16 (55%) 14 (48%) ,0.3: 16 (55%) .0.1 to #0.3 3 (10%) 5 (17%) 0.3-1.0: 7 (24%) .0.3 to #0.5 4 (14%) 3 (10%) .1.0: 6 (21%) .0.5 to #0.6 2 (7%) 1 (3%) Pearson correlation (95% CI) .0.6 to #1.3 1 (3%) 1 (3%) 0.52 (0.19-0.74), R2 = 27% .1.3 (CF or worse) 3 (10%) 5 (17%) Intraclass correlation (95% CI) 0.54 (0.22-0.75) Visual field defect Absolute difference None-minimal (0-0.5) 4 (14%) 8 (28%) ,0.5: 7 (24%) Mild (.0.5-1.0) 4 (14%) 2 (7%) 0.5-1.0: 16 (55%) Moderate (1.5-2.0) 11 (38%) 5 (17%) .1.0: 6 (21%) Marked (2.5-3.0) 5 (17%) 7 (24%) Weighted kappa (95% CI) Severe (3.5-4.0) 5 (17%) 7 (24%) 0.42 (0.21-0.63) CF, counting fingers; IQR, interquartile range; logMAR, logarithm of the minimum angle of resolution. 342 Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. statistics was computed. Overall, 60% (95% CI, 53%- 66%) of patients with bilateral NA-AION had a difference of at least 0.3 logMAR between the first eye and the second eye. The correlation of initial visual acuity between the first eye and the second eye was 0.32 (95% CI, 0.24-0.40) or an R2 of 10.5% (95% CI, 5.7%-16.4%). This indicates that 89.5% of the variation in the visual acuity in the second eye was not explained by its relation to the visual acuity in the first eye. The most recent study on the predictability of visual outcome in 86 patients with sequential episodes of NA-AION was by Mercado et al (9). Their conclusions differed from ours, as they reported, "Visual function between fellow eyes showed a fair to moderate correlation that was statistically significant." This conclusion focused primarily on the P value being ,0.05 instead of the size of the correlation. Although this correlation was statistically sig-nificant, converting this to R2, using the logMAR of the first eye as a predictor of logMAR of the second eye explained only 7.0% of the total variation in the initial visual acuity in the second eye. Their study design also differed from ours with a smaller patient cohort and different method to quan-titate visual field loss. For example, for evaluating visual field loss, they used the method described by Esterman (18), which we found did not provide satisfactory information about quantitative and qualitative changes in visual fields in a study of 101 eyes (11). In addition, pre-existing medical treatment (e.g., aspirin, corticosteroids, pentoxifylline, or bri-monidine tartrate) was not an exclusionary criterion. Moro et al (7) reported that visual acuity in the second eye of bilateral NA-AION cases was slightly better than that in the first eye. In our study, comparison of both initial and final visual acuity between the first eye and the second eye also showed significantly better visual acuity in the second eye (P , 0.0001 for initial and P = 0.030 for final visual acuity). Comparison of the final visual field defect between the first eye and the second eye showed less degree of visual field defect in the second eye (P = 0.004). The reasons for this observation remain unclear. In conclusion, in patients with bilateral sequential NA-AION, there are large differences between visual acuity and visual field findings of paired eyes at both the initial and final visits, with 60% having at least 0.3 logMAR difference between eyes. Regression of the logMAR of the second eye with the logMAR of the first eye explained only 10% of the variation in the logMAR of the second eye. Therefore, it is not possible to predict the visual outcome in the second eye based only on the first eye. REFERENCES 1. Georgiadès G, Konstas P, Stangos N. Study of numerous cases of vascular pseudopapilitis [in French]. Bull Mem Soc Fr Ophthalmol. 1966;79:506-539. 2. Boghen DR, Glaser JS. Ischaemic optic neuropathy: the clinical profile and history. Brain. 1975;98:689-708. 3. Kupersmith MJ, Frohman L, Sanderson M, Jacobs J, Hirschfield J, Ku C, Warren FA. Aspirin reduces the incidence of second eye NAION: a retrospective study. J Neuroophthalmol. 1997;17:250-253. 4. WuDunn D, Zimmerman K, Sadun AA, Feldon SE. Comparison of visual function in fellow eyes after bilateral nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1997;104:104-111. 5. Newman NJ, Scherer R, Langenberg P, Kelman S, Kaufman D, Dickersin K; Ischemic Optic Neuropathy Decompression Trial Research Group. The fellow eye in NAION. Am J Ophthalmol. 2002;134:317-328. 6. Kurz O. Vascular opticopathy. Doc Ophthalmol. 1969;26:582-591. 7. Moro F, Doro D, Mantovani E. Anterior ischemic optic neuropathy and aging. Metab Pediatr Syst Ophthalmol. 1989;12:46-57. 8. Boone MI, Massry GG, Frankel RA, Holds JB, Chung SM. Visual outcome in bilateral nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 1996;103:1223-1228. 9. Mercado JL, Purvin VA, Kawasaki A, WuDunn D. Bilateral sequential nonarteritic anterior ischemic optic neuropathy- a comparison of visual outcomes in fellow eyes using quantitative analysis of Goldmann visual fields. Arch Ophthalmol. 2012;130:863-867. 10. Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625-632. 11. Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy-natural history of visual outcome. Ophthalmology. 2008;115:298-305. 12. Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1029-1046. 13. Hayreh SS, Zimmerman MB. Incipient non-arteritic anterior ischemic optic neuropathy. Ophthalmology. 2007;114:1763- 1772. 14. Hayreh SS, Zimmerman MB. Optic disc edema in non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2007;245:1107-1121. 15. Shrout P, Fleiss J. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420-428. 16. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. 17. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. West Sussex, United Kingdom: John Wiley & Sons, 2009. 18. Esterman B. Grid for scoring visual fields. II. Perimeter. Arch Ophthalmol. 1968;79:400-406. Hayreh and Zimmerman: J Neuro-Ophthalmol 2013; 33: 338-343 343 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |