| OCR Text |
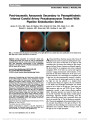
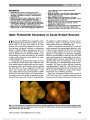
Show Evaluation of Fixation Pattern and Reading Ability in Patients With Leber Hereditary Optic Neuropathy Elke K. Altpeter, MD, Björn R. Blanke, MD, Beate Leo-Kottler, MD, Xuan N. Nguyen, MD, Susanne Trauzettel-Klosinski, MD Background: Leber hereditary optic neuropathy (LHON) is characterized by progressive loss of central vision leading to impaired reading ability. The aim of this study was to evaluate sensory adaptation and reading ability in LHON patients. Methods: This prospective pilot study included 12 male patients with a clinical diagnosis and a positive genetic analysis of LHON, who matched the inclusion criteria of a central scotoma on visual field testing and the use of magnifying aids to read. Examination included best-corrected visual acuity, magnification need, reading speed, and evaluation of fixation by corneal reflexes and by Rodenstock scanning laser ophthalmoscope (SLO). Central scotoma was assessed by conventional perimetry (Tübingen Automated Perimeter) and microperimetry (NIDEK MP1). Results: Mean magnification need was 13.2 ± 7.3-fold (range: 2- to 25-fold). Mean reading speed was 53 ± 18 words per minute (WPM) (range: 24-85 WPM). With auto-mated perimetry, all patients showed central scotomas with a mean radius of 13° ± 7° (range: 1°-30°) in the better eye. Microperimetry in all patients showed fenes-trated central scotomas. Eccentric fixation with a pre-ferred retinal locus (PRL) was detected with SLO examination and microperimetry correlated well in 11 of 12 patients. The SLO results showed no systematic pat-tern in the placement of the PRL; however, 7 of 12 pa-tients (58%) placed their PRL in an unfavorable location left or below the fovea. In 8 of 12 patients, fixation was unstable. Between reading speed and central scotoma size, there was a statistically significant negative corre-lation (P = 0.021, r = 20.65). Conclusions: The percentage of unfavorable PRL locations was extremely high compared with other disorders with central scotomas. Unstable fixation and fenestrated central scotomas led to difficulties in reading. Early rehabilitation and, if necessary, eccentric viewing training should be considered in LHON patients. Journal of Neuro-Ophthalmology 2013;33:344-348 doi: 10.1097/WNO.0b013e31829d1f5b © 2013 by North American Neuro-Ophthalmology Society Leber hereditary optic neuropathy (LHON) leads to pro-found vision loss with a prevalence of 1:31,000 to 1:50,000 (1,2). Mitochondrial point mutations at positions np11778G.A, np3460G.A, and np14484T.C account for most cases (3-5). Despite intense research, no proven therapy for LHON is available. The central visual field loss leads to reading disability at an early stage (6-8), with profound impact on quality of life and independent living (9). Visual rehabilita-tion with magnifying aids is very important for these patients to continue their education and their ability to work (10). The aim of this pilot study was to evaluate the sensory adaptation of LHON patients to their acquired central visual field loss in regard to reading performance. METHODS Patients Thirty-one LHON patients with perimetrically verifiable central scotomas and reading with magnifying aids (optical or electronic) were identified, of whom 12 agreed to participate in our prospective study. All had been using individually optimized low vision aids. The Ethics Com-mittee of the Medical Faculty, University of Tübingen, Germany, approved this study. Informed consent was obtained from all patients. All procedures complied with the tenets of the Declaration of Helsinki. Clinical Examination All patients underwent an ophthalmological examination testing, including best-corrected distance visual acuity (Early Low Vision Clinic and Research Laboratory (EKA, BRB, XNN, ST-K), and University Eye Clinic (BL-K), Center for Ophthalmology, University of Tübingen, Tübingen, Germany. The authors report no conflicts of interest. Address correspondence to Elke K. Altpeter, MD, Low Vision Clinic and Research Laboratory, Center for Ophthalmology, University of Tübingen, Schleichstrasse 12-16, 72076 Tübingen, Germany; E-mail: elke.altpeter@med.uni-tuebingen.de 344 Altpeter et al: J Neuro-Ophthalmol 2013; 33: 344-348 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Treatment Diabetic Retinopathy Study chart), magnification need (assessed as critical print size by the Zeiss chart at 25 cm), and reading speed (IReST cards = International Reading Speed Texts) (11) with the individual magnifying aid (the magnifying aid was provided according magnification need of critical print size). We performed conventional perimetry (Tübingen Auto-mated Perimeter 30°) and microperimetry with the NIDEK MP1 (Nidek, Padua, Italy). The MP1 provides automated full-threshold perimetry with fundus tracking to correct for eye movements. Microperimetry and examination by scanning laser ophthal-moscope (SLO) fixation behavior were always assessed monoc-ularly. To assess fixation behavior binocularly, the location of corneal reflexes was measured by an orthoptic examination, the "Hirschberg" test (12). One millimeter decentration of the corneal reflex equated to 7° deviation of gaze. Examination by Scanning Laser Ophthalmoscope and Preferred Retinal Locus Patients with an absolute central scotoma fixate eccentrically. This eccentric area is called "preferred retinal locus" (PRL) (13) and becomes the new sensory and oculomotor "center" (14). The same PRL may be used for reading but can also be located elsewhere depending on the reading visual field (15). The location of the PRL was identified by SLO (SLO 101; Rodenstock Instruments, Munich, Germany). From the PRL in the SLO image of the retina, we determined the equivalent location of the PRL in the visual field. For example, fixation above the fovea detected with the SLO corresponds to a fix-ation locus (FL) below the scotoma in the visual field. Using the SLO, patients fixated on a central cross (36 arc minutes in diameter) with the better eye were recorded on videotape. The better eye was defined as the eye with the lower magnification need. Fixation stability was assessed semiquantitatively by tracking a retinal blood vessel on the SLO image for 15 seconds on an overhead transparency by a text marker. The quality of the SLO images was not sufficient for a quantitative analysis at the time of this study. The size of the fixation area was measured semiquantitatively in relation to the size of the optic disc. Statistical Analysis We calculated the means, standard deviations, and ranges from the individual values. Correlations between reading speed and scotoma size were calculated using Pearson correlation coefficient. P-values ,0.05 were regarded as indicators of statistical significance. Statistical analysis was performed using "R," an open source programming language for statistical com-puting, version 2.15.1 (http://www.revolutionanalytics.com/). RESULTS Mean age of our patient cohort at the time of examination was 41 years (range: 20-70 years). Mean duration since onset of disease was 18 years (range: 2-44 years). Seven patients had the mutation np11778G.A, 4 patients np3460G.A, and 1 patient np14484T.C. Mean best-corrected distance visual acuity with the better eye was 1.38 logarithm of the minimum angle of resolution (range: 0.5-2.0 logarithm of the minimum angle of resolution; range in Snellen range: 20/40 to 20/200). Mean magnification required by our pa-tients was 13.2-fold (range: 2- to 25-fold). Mean reading speed was 53 ± 18 words per minute (WPM) (range: 24-85 WPM). Ten patients used an electronic magnifying device, and 2 patients used magnifying spectacles. In testing binocular ocular alignment by corneal reflexes, 4 patients showed fixa-tion of 5° to 15° upgaze, 6 patients had near central fixation, and 2 patients fixated in 5° to 15° downgaze. Clinical findings are summarized in Table 1 and an illustrative case is presented in Figure 1. Evaluation of Central Scotomas Using automated perimetry, all patients showed absolute central scotomas. In the better eye, mean radius of the central TABLE 1. Summary of clinical findings in patients with Leber hereditary optic neuropathy Patient Visual Acuity (LogMAR) Magnification Need Magnifying Aid Reading Speed (WPM) Scotoma Radius (TAP30°) 1 1.7 12.5 em 56 15 2 1.0 16.0 em 85 8 3 2.0 25.0 em 24 30 4 1.1 6.25 em 46 10 5 1.1 6.25 em 56 15 6 1.5 8.0 em 40 8 7 2.0 20.0 em 35 15 8 1.7 20.0 em 40 15 9 1.4 16.0 em 67 10 10 1.6 20.0 em 46 15 11 0.5 2.0 ms 75 1 12 1.0 6.25 ms 63 10 em, electronic magnifier; logMAR, logarithm of the minimum angle of resolution; ms, magnifying spectacles; TAP, Tübingen Automated Perimeter; WPM, words per minute. Altpeter et al: J Neuro-Ophthalmol 2013; 33: 344-348 345 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. scotoma was 13° ± 7° (range: 1°-30°). All microperimetry results with the NIDEK MP1 showed a fenestrated central scotoma, that is the central scotoma was not completely dense, but there were tiny "holes" or "isles" in the scotoma with better retinal function (16,17). Fixation Behavior Analyzed by SLO and MP1 Microperimetry Fixation Locus During fixation of a central cross with the better eye, the SLO showed the PRL above or diagonally above the fovea in 4 eyes (i.e., fixation was below the central scotoma in the visual field), and in right of the fovea in 1 eye. A PRL right of the scotoma is less favorable than left because it is not in the direction of reading. In 4 eyes, the PRL was left of the fovea/ central scotoma, and in 3 eyes, the PRLs lay below or diagonally below the fovea (i.e., above the central scotoma in the visual field). The remaining 7 eyes (58%) showed an unfavorable PRL for reading located below and left of the fovea. Eleven patients showed correspondence between the FL in MP1 microperimetry and in the SLO. The PRL in the SLO was different from the PRL in microperimetry in only Patient 8. In 7 of 12 patients, the monocular fixation behavior in the better eye using the SLO matched the fixation behavior detected with the binoc-ular orthoptic examination. It did not match for patients 3, 7, 8, 10, and 12. Fixation Stability In 8 of 12 patients, fixation was very unstable with large fixation areas (Fig. 2). FIG. 1. Testing results of left eye in Case 2. A. Automated perimetry shows the blind spot and central scotoma shifted to the left and slightly upwards (arrow). B. Microperimetry plot reveals a fenestrated central scotoma. C. Eccentric fixation on a central cross is found with scanning laser ophthalmoscope (preferred retinal locus above right). D. Gaze direction is upward 5° to the left. There is good agreement of fixation behavior in this patient determined by 4 different methods. 346 Altpeter et al: J Neuro-Ophthalmol 2013; 33: 344-348 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Reading Speed There was a statistically significant negative correlation (Pearson product-moment) between reading speed and the size of the central scotoma determined with automated perimetry, that is, the larger the scotoma, the lower the reading speed (correlation coefficient r = 20.65, df = 10, P = 0.021). Patients with a reading speed below 50 WPM showed an unfavorable PRL and/or had high magnification requirements. DISCUSSION This is the first systematic study examining fixation behavior and reading speed in patients with LHON. Our finding of fenestrated scotomas in patients with LHON is consistent with previous reports (16,17). In all 12 patients, we demonstrated that the PRL was located within the sco-toma. The fenestrations were too small for reading, not meeting the minimum size of 2° to the left and right of fixation (10,18,19). Additionally, the size of the central scotoma in our patients was quite large with a mean radius of 13° ± 7°. Therefore, our patients had very high magni-fication need and very poor reading speed. This resulted in a negative correlation between scotoma size and reading speed. In our LHON patients, our findings of an average magnification need of 13.2-fold and an average reading speed of 53 WPM are comparable with patients with large central scotomas due to advanced age-related macular degeneration. In a study of 298 patients with advanced FIG. 2. Fixation stability of a single cross was measured for the better eye in all patients by SLO. The fixational eye movements are shown in red in the second column. They were assessed by semiquantitative tracking of a fundus landmark. The size of the fixation area was measured semi-quantitatively in relation to the optic disc size (3rd column). Stable fixation shows a small red area (patients # 4, 5, 11, 12), unstable fixation shows more drifts and jumps of the red line and a larger area. The patients are listed by reading speed. The last 6 patients show reading speeds below 50 WPM, have a high magnification need and/or an unfavorable PRL location below or left of the fovea. em, electronic magnifier; logMAR, logarithm of the minimum angle of resolution; ms, magnifying spectacles; PRL, preferred retinal locus; SLO, scanning laser ophthalmoscope; WPM, words per minute. Altpeter et al: J Neuro-Ophthalmol 2013; 33: 344-348 347 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. AMD, if magnification need was more than 6-fold, their reading speed using standardized texts (IReST) was poor at 46 ± 20 WPM (20). We used both SLO and microperimetry to examine each patient's PRL. We found good correlation of these 2 two methods, which is in agreement with other studies (21,22). Some patients choose a PRL location that is favorable for orientation and reading and some choose an unfavorable one. Besides the topography of the central scotoma, additional factors play a role, including focal sustained attention (23). A variety of reports have examined PRL distribution in different locations in patients with central scotomas. These scotomas arise from a variety of causes, including AMD and macular dystrophies. In most cases (39%-76%), the central scotoma is located above the FL, that is, the scotoma is shifted upward, optimal for daily tasks of living, especially reading. In 16% to 34% of cases, fixation was left of the fovea, that is, the central scotoma is on the right side of the FL (i.e., the scotoma is shifted to the right, in the reading direction); in 5% to 19.9% of cases, it was shifted to the left, and in 2.5% to 7.5% of cases, it was shifted downwards (24-27). A scotoma below the FL will lead to difficulties with walking and reading. Fixation left of the fovea means shifting the scotoma in the reading direc-tion (i.e., left to right). Our LHON patients often chose an unfavorable PRL location: 3 below the fovea and 4 left of the fovea. The unfavorable location of the PRL coupled with unstable fixation and fenestrated central scotomas all contributed to difficulty reading. Limitations of our study include small sample size and use of Zeiss charts to determine critical print size. These charts have yet to be validated for establishing critical print size. Another limitation is long duration of vision impair-ment in most of our patients, which likely resulted in long established PRLs. Possibly early intervention with low vision services combined with eccentric reading training in LHON patients will lead to improvement in both reading speed and quality of life. REFERENCES 1. Man PY, Griffiths PG, Brown DT, Howell N, Turnbull DM, Chinnery PF. The epidemiology of Leber hereditary optic neuropathy in the North East of England. Am J Hum Genet. 2003;72:333-339. 2. Puomila A, Hämäläinen P, Kivioja S, Kivioja S, Savontaus M-L, Koivumäki S, Huoponen K, Nikoskelainen E. Epidemiology and penetrance of Leber hereditary optic neuropathy in Finland. Eur J Hum Genet. 2007;15:1079-1089. 3. Huoponen K. Leber hereditary optic neuropathy: clinical and molecular genetic findings. Neurogenetics. 2001;3: 119-125. 4. Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988;242:1427-1430. 5. Johns DR, Heher KL, Miller NR, Smith KH. Leber's hereditary optic neuropathy: clinical manifestations of the 14484 mutation. Arch Opthalmol. 1993;111: 495-498. 6. Newman NJ. Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol. 2005;140:517-523. 7. Leo-Kottler B, Wissinger B. Lebersche Optikusatrophie. Ophthalmologe. 2011;108:1179-1192. 8. Newman NJ. Hereditary optic neuropathies. In: Miller NR, Newman NJ, Biousse V, Kerrison JB, eds. Walsh & Hoyt's Clinical Neuro-ophthalmology, 6th edition. Baltimore, MD: Williams & Wilkins, 2005,465-501. 9. Kirkman MA, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, Klopstock T, Griffith PG, Husdon G, Chinney PF, Yu-Wai-Man P. Quality of life in patients with Leber hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2009;50:3112-3115. 10. Trauzettel-Klosinski S. Rehabilitation for visual disorders. J Neuroophthalmol. 2010;30:73-84. 11. Trauzettel-Klosinski S, Dietz K; The IReST Study Group. Standardized Assessment of Reading Performance: the New International Reading Speed Texts IReST. Invest Ophthalmol Vis Sci. 2012;53:5452-5461. 12. Hasebe S, Ohtsuki H, Kono R, Nakahira Y. Biometric confirmation of the Hirschberg ratio in strabismic children. Invest Ophthalmol Vis Sci. 1998;39:2782-2785. 13. Timberlake GT, Peli E, Essock EA, Augliere RA. Reading with a macular scotoma. II. Retinal locus for scanning text. Invest Ophthalmol Vis Sci. 1987;28:1268-1274. 14. White JM, Bedell HE. The oculomotor reference in humans with bilateral macular disease. Invest Ophthalmol Vis Sci. 1990;31:1149-1161. 15. Fletcher DC, Schuchard RA, Watson G. Relative locations of macular scotomas near the PRL: effect on low vision reading. J Rehabil Res Dev. 1999;36:356-364. 16. Kerrison JB, Newman NJ. Clinical spectrum of Leber's hereditary optic neuropathy. Clin Neurosci. 1997:4:295-301. 17. Mashima Y, Sato EA, Ohde H, Oguchi Y. Macular nerve fibers temporal to fovea may have a greater potential to recover function in patients with Leber's hereditary optic neuropathy. Jpn J Ophthalmol. 2002;46:660-667. 18. Aulhorn E. Über Fixationsbreite und Fixationsfrequenz beim Lesen gerichteter Konturen. Pflügers Arch. 1953;257:318-322. 19. Legge GE, Ahn SJ, Klitz TS, Lvebkr A. Psychophysics of reading. XVI. The visual span in normal and low vision. Vision Res. 1997;37:1999-2010. 20. Nguyen NX, Trauzettel-Klosinski S. Effectiveness of magnifyling low vision aids in patients with age-related macular degeneration. Neuroopthalmology. 2009;33:115-119. 21. Rohrschneider K, Springer C, Bültmann S, Völcker HE. Microperimetry comparison between the micro perimeter 1 and scanning laser ophthalmoscope-fundus perimetry. Am J Ophthalmol. 2005;139:125-134. 22. Dunbar HM, Crossland MD, Rubin GS. Fixation stability: a comparison between the Nidek MP-1 and the Rodenstock scanning laser ophthalmoscope in persons with and without diabetic maculopathy. Invest Ophthalmol Vis Sci. 2010;5:4346-4350. 23. Altpeter E.K., Mackeben M., Trauzettel-Klosinski S. The importance of sustained attention for patients with maculopathies. Vis Res. 2000;40:1539-1547. 24. Messias A, Reinhard J, Velasco e Cruz AA, Dietz K, Mackeben M, Trauzettel-Klosinski S. Eccentric fixation in Stargardt's disease assessed by Tübingen perimetry. Invest Opthalmol Vis Sci. 2007;48:5815-5822. 25. Fletcher DC, Schuchard RA. Preferred retinal loci relationship to macular scotomas in a low-vision population. Ophthalmology. 1997;104:632-638. 26. Guez JE, Le Gargasson JF, Rigaudiere F, O'Regan JK. Is there a systematic location for pseudo-fovea in patients with central scotoma. Vis Res. 1993;33:1271-1279. 27. Trauzettel-Klosinski S, Tornow R. Fixation behavior and reading ability in macular scotoma: assessed by Tübingen manual perimetry and scanning laser ophthalmoscopy. Neuroophthalmology. 1996;16:241-253. 348 Altpeter et al: J Neuro-Ophthalmol 2013; 33: 344-348 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |