| OCR Text |
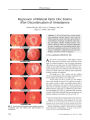
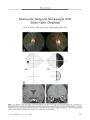
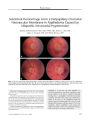
Show ORIGINAL CONTRIBUTION Small Cell Lung Carcinoma Presenting as Collapsin Response- Mediating Protein ( CRMP) - 5 Paraneoplastic Optic Neuropathy Rishi Sheorajpanday, MD, Hans Slabbynck, MD, Wivine Van De Sompel, MD, Danny Galdermans, MD, Ingrid Neetens, MD, and Peter Paul De Deyn, MD, PhD Abstract: A 77- year- old woman presenting with progressive visual loss in both eyes was found to have small cell lung cancer. Assay for collapsin response-mediating protein ( CRMP) - 5 was positive suggesting a paraneoplastic optic neuropathy ( PON). During treatment of the small cell lung cancer, the patient died of pneumonia and autopsy disclosed neuropath-ologic abnormalities consistent with PON. This is only the second case of CRMP- 5- confirmed PON to report neuropathologic findings. (/ Neuro- Ophthalmol 2006; 26: 168- 172) Paraneoplastic optic neuropathy ( PON) is a rare cause of visual loss. Approximately 25 cases have been reported, in 19 of which collapsin response- mediating protein ( CRMP) - 5, a 62- kDa protein, has been identified ( 1). Neuropathologic findings have been described in 6 cases of PON, including only one case involving CRMP- 5 ( 1). We present a case of isolated bilateral progressive visual loss in the setting of small cell lung carcinoma that was diagnosed as PON with CRMP- 5 serology and supplemented with autopsy neuropathologic findings. Departments of Neurology and Memory Clinic ( RS, PPD), Pneumology ( HS, DG), Ophthalmology ( WV), and Pathology ( IN), Middelheim General Hospital, Antwerp, Belgium; and the Laboratory of Neurochemistry and Behaviour, Institute Born- Bunge, Department of Biomedical Sciences ( RS, PPD), University of Antwerp, Edegem, Belgium. Address correspondence to Prof. Peter Paul De Deyn, MD, PhD, University of Antwerp, Middelheim Hospital, ZNA, Department of Neurology Lindendreef 1,2020 Antwerp, Belgium; E- mail: peter. dedeyn@ ua. ac. be CASE REPORT A 77- year- old woman was admitted to our neurologic ward because of progressive painless visual loss of the right eye for 2 months. Visual acuity in the left eye had been documented at finger counting for several years. Our examination showed finger counting acuity in both eyes. Direct and consensual pupil reactions were normal in the right eye. In the left eye, however, the pupil light reaction was very weak. Biomicroscopic examination was normal apart from bilateral cataract. Applanation tonometry was 16 and 19 mm Hg. Ophthalmoscopy of the right eye revealed a swollen optic disc with ill- defined margins and multiple peripapillary hemorrhages with a normal- appearing macula ( Fig. 1). Ophthalmoscopy of the left eye disclosed a pale, indistinct optic disc. Within one week, visual acuity deteriorated to light perception in the right eye and to hand movements in the left eye. Ophthalmoscopy showed small vitreous cells. There were cotton wool spots at the inferior margin of the right optic disc. There were no cells in the anterior chamber. Ophthalmoscopic examination of the left eye was unchanged. Neurologic examination was otherwise normal. A chest x- ray revealed a 5.5 X 5.5- cm mass in the right upper lobe. CT disclosed the mass in the absence of mediastinal invasion or lymphadenopathy ( Fig. 2). Complete blood count, electrolytes, and acute- phase reactants were normal. Whole- body fluorodeoxyglucose positron emission tomography ( FDG- PET) suggested tumor activity in the apex of the right lung, the right parotid-submandibular junction, and in the left nasopharynx. CT suggested a benign salivary gland tumor. Nasopharyngeal mucosal biopsy was negative for neoplasm. Needle biopsy of the lung lesion disclosed undifferentiated small cell carcinoma ( SCLC). Brain MRI was normal except that it showed hyperintense signal on T2 imaging and mild enhancement of the right optic nerve ( Fig. 3). 168 J Neuro- Ophthalmol, Vol. 26, No. 3, 2006 CRMP- 5 Paraneoplastic Optic Neuropathy J Neuro- Ophthalmol, Vol. 26, No. 3, 2006 FIG. 1. Bedside fundus photography shows marked optic disc edema in the right eye with papillary hemorrhages and a pale, indistinct optic disc in the left eye. FIG. 2. Chest CT shows a large mass ( arrow) in the right upper lobe that proved to be small cell carcinoma. Lumbar puncture showed a normal opening pressure and acellular fluid with a normal glucose but an elevated protein at 84 mg/ dL. Cytology was negative. There was an oligoclonal banding pattern in the gamma globulin region; by isoelecrofocusing, 7 cerebrospinal fluid ( CSF)- specific oligoclonal fractions between pH 7 and 8.6 were detected. Duplex imaging showed normal flow in both ophthalmic arteries. Anti- neuronal antibody assay was positive for anti- CRMP- 5 but not for anti- Hu, anti- Yo, anti- Ri, anti-amphysine, anti- Ma, and anti- Tr, and was negative for anti-calcium channel antibodies. Cisplatinum- etoposide chemotherapy and radiotherapy for the lung tumor resulted in decreased tumor volume on subsequent CT. Visual acuity, however, deteriorated further to light perception bilaterally. Ophthalmoscopic examination after 2 months revealed bilateral disc pallor without swelling and with small- caliber retinal vessels. Neurologic examination 2 and 4 months later still disclosed no other abnormalities. Four months after presentation, the patient died of overwhelming pneumonia. General autopsy disclosed an irregularly margined tumor with a maximum diameter of 1.5 cm in the right upper lung lobe. Microscopic examination revealed extensive regions of necrosis with foamy macrophages, lymphocytes, monocytes, and plasma cells but no viable tumor cells. There were no macroscopically detectable metastases. Neuropathologic examination, limited to the optic nerves, disclosed thickening of the right optic nerve with reactive changes consisting of spongiosis, perivascular edema, and perivascular lymphocytic infiltration mostly positive for CD3 and CD20 without any signs of metastasis ( Fig. 4). These findings were interpreted as consistent with chronic PON. FIG. 3. A. Postcontrast T1 coronal MRI demonstrates a relatively enlarged and enhancing right optic nerve ( arrow) as compared with the left optic nerve ( arrowhead). B. T2 coronal MRI shows that the right optic nerve has relatively high signal compared with the left optic nerve. 169 J Neuro- Ophthalmol, Vol. 26, No. 3, 2006 Sheorajpanday et al FIG. 4. Autopsy findings. A. Cross- section of the swollen right optic nerve shows spongiosis and perivascular lymphocytic infiltration. B. Cross- section of the left optic nerve shows minimal changes ( hematoxylin and eosin, scale bar = 1,000 | xm). C. Microscopic section of the right optic nerve shows prominent spongiosis ( S) and perivascular lymphocytic infiltration ( arrow). D. Similar but much less evident changes are present in the left optic nerve ( hematoxylin and eosin, scale bar = 100 | xm). E. Immunohistochemistry for the pan- T- cell marker CD3 of the right optic nerve is clearly positive ( scale bar = 200 | xm). F. Immunohistochemistry for CD20 in the same right optic nerve area is also positive ( scale bar = 200 | xm). 170 © 2006 Lippincott Williams & Wilkins CRMP- 5 Paraneoplastic Optic Neuropathy J Neuro- Ophthalmol, Vol. 26, No. 3, 2006 DISCUSSION In the estimated 25 reported cases of PON, most patients have also displayed ophthalmoplegia ( 2- 5), retinitis, multifocal neurologic deficits ( 1), or a cerebellar syndrome ( 6- 9). Our patient with CRMP- 5 PON is unusual in presenting with isolated bilateral optic neuropathy after which a diagnosis of SCLC was made. A seemingly similar case of isolated PON in previously diagnosed non- small cell lung carcinoma was recently reported ( 10). In this case, however, the diagnosis was not confirmed with anti-neuronal antibody. PON produces unilateral or bilateral progressive visual loss with optic disc edema followed by disc pallor and accompanied by lymphocytosis and protein elevation in the CSF ( 11). In our patient, ophthalmoscopic findings initially suggested the possibility of intraocular lymphoma for which a diagnostic vitrectomy was planned. However, once the lung mass was detected, we considered PON and elected to draw an anti- neuronal antibody assay. Once anti- CRMP- 5 antibody was confirmed, vitrectomy was not necessary. Hoh et al ( 12) described a case of PON in nasopharyngeal carcinoma ( 12), but pathologic examination of the nasopharynx in our patient revealed no neoplasia. Anti- CRMP- 5 antibodies are most commonly associated with SCLC. Also implicated are other types of bronchial carcinoma, nasopharyngeal carcinoma, Hodgkin and non- Hodgkin lymphoma, neuroblastoma, pancreatic glucago-noma, and thymoma ( 13). The pathophysiological mechanism by which anti- CRMP- 5 antibodies cause optic neuropathy remains unclear. Because CRMP- 5 is expressed in SCLC ( 1,13,14), the most plausible pathogenetic mechanism of PON in SCLC is an immune response against the onconeural antigen. There is still no adequate pathogenetic explanation for the wide range of neurologic manifestations with which CRMP- 5- seropositive patients present ( optic neuropathy, other cranial neuropathies, retinitis, cerebellar degeneration, diverse forms of encephalomyelitis [ 1,13]). Of the 116 CRMP- 5- seropositive patients reported by Yu et al ( 14), 8 had optic neuropathy; in only 3 of these cases was optic neuropathy the initial presentation. Therapy of paraneoplastic syndromes is often directed at the tumor, although a significant number of patients experience irreversible damage. The neurologic manifestations often remit, although not completely, after treatment of the malignancy. In our patient, there was a rather rapid evolution from optic disc swelling to atrophy under chemotherapy. Direct treatment of the paraneoplastic syndrome with intravenous immunoglobulin, not tried in our patient, has produced mixed results ( 15). It is not useful in severely disabled patients with anti-neuronal antibodies ( 16). Neuropathologic examination in our patient was typical of previously described cases of PON ( 1,2,5,6). Of special interest are the findings described by Cross et al ( 1) in the only other CRMP- 5- positive PON with neuropathologic examination. Histopathologic examination of the eye and optic nerve from that patient showed chronic optic neuritis with inflammatory infiltrates composed predominantly of T cells, most of which were CD8 + . Patchy loss of axons and myelin was associated with the inflammatory infiltrates ( 1). That patient, with metastatic small cell carcinoma in a hilar lymph node, however, also had dementia, partial complex seizures, limited upgaze, and myelopathy with numerous white matter lesions on brain MRI and a patchy high- intensity T2 signal on spinal cord MRI. Neuropathologically, there was marked involvement of medial temporal structures ( particularly amygdala), cerebellum, brainstem, and spinal cord. Mild loss of myelinated axons was noted in peripheral nerves, spinal rootlets, and spinal sensory ganglia at all levels ( 1). Most interestingly, that patient also harbored anti- N- type calcium channel antibodies ( 1). It is possible that these anti- N- type calcium channel antibodies contributed to the findings in that patient. Additional analysis for anti- calcium channel antibodies was negative in our patient. Perhaps our case is the first PON with neuropathologic findings attributable to anti- CRMP- 5 antibodies alone. Further research is necessary to elucidate the exact nature of anti- CRMP- 5- associated PON. REFERENCES 1. Cross SA, Salamao DR, Parisi JE, et al. Paraneoplastic autoimmune optic neuritis with retinitis defined by CRMP- 5- IgG. Ann Neurol 2003; 54: 38- 50. 2. Pillay N, Gilbert JJ, Ebers GC, et al. Internuclear ophthalmoplegia and ' optic neuritis': paraneoplastic effects of bronchial carcinoma. Neurology 1984; 34: 788- 91. 3. Waterston JA, Gilligan BS. Paraneoplastic optic neuritis and external ophthalmoplegia. Aust N Z J Med 1986; 16: 703^ k 4. Anderson NE. Paraneoplastic optic neuritis and external ophthalmoplegia. AustN Z J Med 1987; 17: 539. 5. Boghen D, Sebag M, Michaud J. Paraneoplastic optic neuritis and encephalomyelitis. Report of a case. Arch Neurol 1988; 45: 353- 6. 6. Malik S, Furlan AJ, Sweeney PJ, et al. Optic neuropathy: a rare paraneoplastic syndrome. J Clin Neuroophthalmol 1992; 12: 137^ 1. 7. De la Sayette Y Bertran F, Ffonnorat J, et al. Paraneoplastic cerebellar syndrome and optic neuritis with anti- CV2 antibodies: clinical response to excision of the primary tumor. Arch Neurol 1998; 55: 405- 8. 8. Luiz JE, Lee AG, Keltner JL, et al. Paraneoplastic optic neuropathy and autoantibody production in small- cell carcinoma of the lung. J Neuroophthalmol 1998; 18: 178- 81. 9. Thambisetty MR, Scherzer CR, Yu Z, et al. Paraneoplastic optic neuropathy and cerebellar ataxia with small cell carcinoma of the lung. J Neuroophthalmol 2001; 21: 164- 7. 10. Asproudis IC, Nikas AN, Psilas KG. Paraneoplastic optic neuropathy in a patient with a non- small cell lung carcinoma: a case report. Eur J Ophthalmol 2005; 15: 420- 3. 11. Ling CP, Pavesio C. Paraneoplastic syndromes associated with visual loss. Curr Opin Ophthalmol 2003; 14: 426- 32. 171 J Neuro- Ophthalmol, Vol. 26, No. 3, 2006 Sheorajpanday et al 12. Hoh ST, Teh M, Chew SJ. Paraneoplastic optic neuropathy in nasopharyngeal carcinoma- report of a case. Singapore Med J1991; 32: 170- 3. 13. Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol 2003; 48: 12- 38. 14. Yu Z, Kryzer TJ, Griesmann GE, et al. CRMP- 5 neuronal autoantibody: marker of lung cancer and thymoma related autoimmunity. Ann Neurol 2001; 49: 146- 54. 15. Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol 1999; 117: 471- 7. 16. Keime- Guibert F, Graus F, Fleury A, et al. Treatment of paraneoplastic neurologic syndromes with antineuronal antibodies ( anti- Hu, anti- Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry 2000; 68: 479- 82. 172 © 2006 Lippincott Williams & Wilkins |