| OCR Text |
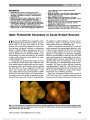
Show First Cases of Dominant Optic Atrophy in Saudi Arabia: Report of Two Novel OPA1 Mutations Alberto Galvez-Ruiz, PhD, Christine Neuhaus, PhD, Carsten Bergmann, PhD, Hanno Bolz, PhD Background: Fifty to 60% of patients with dominant optic atrophy (DOA) have mutations of the OPA1 gene, which enc-odes dynamin-related GTPase, a protein of the internal mito-chondrial membrane. To date, more than 200 OPA1 mutations in the OPA1 gene have been described. However, DOA is genetically heterogeneous with certain families linked to other chromosomal loci, that is, OPA3, OPA4, OPA5, and OPA7. Methods: This study describes a clinical series of 40 patients from Saudi Arabia with a positive DOA phenotype (i.e., decreased visual acuity during the first 2 decades of life, temporal or global optic disc pallor, and absence of other neurological or ophthalmological diseases that could explain the optic neuropathy) who underwent molecular genetic testing for OPA1 (and, in some cases, for OPA3). Results: This study describes for the first time 4 OPA1 muta-tions in DOA patients from Saudi Arabia, including 2 novel OPA1 mutations in 2 different patients. Conclusion: The question remains whether certain patients in Saudi Arabia with a clearly defined DOA phenotype may be due to mutations in chromosomal loci other than OPA1 and OPA3. It is likely that genetic alterations associated with different loci will be discovered in the future. Journal of Neuro-Ophthalmology 2013;33:349-353 doi: 10.1097/WNO.0b013e31829ffb9a © 2013 by North American Neuro-Ophthalmology Society The hereditary optic neuropathies most frequently described in the scientific literature are Leber hereditary optic neuropathy (LHON) and dominant optic atrophy (DOA), both resulting from mitochondrial disorders (1). Mitochondrial diseases may be caused either by mutations of the mitochondrial DNA (mtDNA), as in LHON, or by mutations of nuclear genes encoding proteins of the mito-chondrion. For example, the major DOA gene, OPA1, is part of the nuclear genome, and OPA1-related DOA is inherited in a Mendelian fashion, as an autosomal dominant trait (1-3). The majority of OPA1 mutations (up to 50%) are trun-cated, predicting either shortened OPA1 protein or haploin-sufficiency because of nonsense-mediated decay of the mutant transcript. However, missense mutations preserving OPA1 protein have been described (1,3). These mutations affect the stability of mitochondria and the function of the mitochondrial respiratory chain (4,5). Clinically, DOA manifests as a progressive loss of visual acuity (VA) that begins in the first 2 decades of life with temporal pallor of the optic discs and cecocentral scotomas on visual field testing. The extent of VA deterioration does not tend to be as pronounced as in LHON patients; however, there is a significant phenotypic overlap between DOA and LHON (1,6-8). In addition, a "DOA plus" phenotype has been reported, which includes deafness, ataxia, myopathy, and peripheral neu-ropathy (1,9). This suggests that clinical expressivity of OPA1 mutations is variable and other cell groups may be affected. In this study, we report the results of clinical and molecular evaluation in a series of 40 DOA patients from Saudi Arabia. MATERIALS AND METHODS Patients Forty patients from different regions of Saudi Arabia were referred to a tertiary care center (King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia) between 2010 and 2012 evaluation of optic neuropathy present in the first 2 decades of life. Inclusion criteria were as follows: VA decrease in the first 2 decades of life, temporal or diffuse pallor of the optic disc, and absence of other neurological Neuro-Ophthalmology Division (AG-R), King Khaled Eye Specialist Hospital, Riyadh, Kingdom of Saudi Arabia; Center for Human Genetics (CN, CB, HB), Bioscientia, Ingelheim Germany; Center for Clinical Research (CB), University Hospital of Freiburg, Freiburg, Germany; and Institute of Human Genetics (HB), University Hospital of Cologne, Cologne, Germany. The authors report no conflicts of interest. Address correspondence to Alberto Galvez-Ruiz, PhD, PO Box 7191, Riyadh 11462, Kingdom of Saudi Arabia; E-mail: algarui@yahoo.com Galvez-Ruiz et al: J Neuro-Ophthalmol 2013; 33: 349-353 349 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. and ophthalmological diseases that could explain the optic neuropathy (e.g., demyelinating disease, perinatal hypoxia, previous trauma). Patients with nystagmus and strabismus were included. Although the autosomal dominant inheritance of OPA1-related DOA often implies a multigenerational ped-igree, patients with DOA phenotype, but without family history of VA loss, also were enrolled. Medical and family history was recorded for all patients; however, in some pa-tients, these data could not be reliably obtained. All patients were evaluated in the Division of Neuro- Ophthalmology by the same neuro-ophthalmologist (A.G.-R.) with the following tests: VA, color vision (Ishihara color plates), kinetic visual fields (KVF), (if the patient's age allowed for adequate cooperation). In addition, patients underwent neuro-logical examination and magnetic resonance imaging (MRI) of the brain and orbits. The research protocol had been approved by the locally appointed ethics committee (King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia) and informed con-sent was obtained from subjects (or their guardians). Genetic Analysis A blood sample from all 40 patients was used for genetic testing for OPA1, whereas OPA3 was analyzed in 23 patients. The 30 coding exons and the exon-intron boundaries of OPA1 on chromosome 3q28-29 (OMIM 605290) were amplified by polymerase chain reaction (PCR) and sequenced directly. The resulting sequence data were compared with the reference sequence NM_130837.2. We also conducted a deletion analysis of OPA1 (OMIM 605290) with the material obtained by multiplex ligation-dependent probe amplification (MLPA); kit: SALSA MLPA P229-B1 OPA1 (10). All coding exons of OPA1 and the adjacent regions were screened for deletions/duplications. To identify OPA3 mutations, the 2 coding exons and the exon-intron boundaries of the OPA3 gene on chromosome 19q13.32 (OMIM 606580) were amplified by PCR and sequenced directly. The resulting sequence data were com-pared with the reference sequence NM_025136.3. Additionally, given the clinical overlap between patients with DOA and LHON, the 3 most frequent genetic LHON mutations at mtDNA positions 11,778, 3460, and 14,484 were excluded in all patients. For these analyses, mtDNA was obtained from peripheral blood leukocytes. Portions of the ND-4, ND-1, and ND-6 genes were amplified using the PCR-based amplification-refractory mutation system. RESULTS Our case series consisted of 40 patients, 27 men (67.5%) and 13 women (32.5%), with a mean age of 22.35 years (range, 7-55 years). Sixteen patients (40%) had a positive family history of optic nerve disease and 25 (62.5%) had consan-guineous parents. VA ranged from 20/20 to 20/400 (mean: 20/160). Using Ishihara color plates, color vision ranged from 0/15 to 15/15 plates read correctly (mean: 6/15). Twenty-seven patients had temporal optic disc pallor and 13 (32.5%) had diffuse pallor. On KVF testing, most patients exhibited cecocentral scotomas (50%) followed by arcuate defects (12.5%) and generalized decreased sensitivity (12.5%), and 10% had normal visual fields. Nine patients (22.5%) presented with strabismus and/or nystagmus. The range of eye movements was normal in all these patients, and nystagmus was horizontal and pendular. Six patients (15%) had hearing loss or unsteady gait (DOA plus syndrome), and 38 patients (95%) showed no disease progression during the 2 years of follow-up. Genetic tests for OPA1 and LHON were conducted for all patients while OPA3 was analyzed in 23 patients. No LHON or OPA3 mutations were identified. Four patients (10%) were identified with mutations in OPA1. Case Reports Case 1 A 12-year-old girl was referred to neuro-ophthalmology because of mild, bilateral optic disc pallor. The patient was asymptomatic and family history was unremarkable. Her parents were distantly related (no first-degree consanguinity). VA was 20/30, right eye, and 20/25, left eye and the patient was able to identify 12 of 15 color plates with the right eye and 11 of 15 with the left eye.Minimal temporal pallor of the optic discs was detected on funduscopy (Fig. 1). KVF and brain MRI were within normal limits. An OPA1 gene sequence analysis was performed that revealed the heterozygous mutation c.852T.A, substitut-ing the codon for tyrosine by a stop codon at position 284 (p.Tyr284X). This nonsense mutation either results in mes-senger RNA degradation (nonsense-mediated decay) or in a truncation of the OPA1 protein. Case 2 A 17-year-old adolescent boy had a history of bilateral progressive VA loss since early childhood. There was no family history of ophthalmic or neurologic disease. VA was 20/80, right eye, and 20/60 left eye, and the patient could identify 9 of 15 color plates with each eye. There was temporal pallor of both optic nerves (Fig. 1). KVF and brain and orbital MRI were unremarkable. OPA1 gene sequencing revealed the heterozygous muta-tion c.1099C.T, substituting the codon for arginine by a stop codon at position 367 (p.Arg367X). This nonsense mutation results in nonsense-mediate decay or in a trunca-tion of the OPA1 protein and can be regarded pathogenic. Case 3 A 13-year-old boy presented with a history of bilateral visual loss beginning early in life. There was no significant family history of eye disease, but there was consanguinity between his parents, although not of first degree. VA was 20/100 bilaterally and he was able to see 1 of 15 color plates with the right eye and 4 of 15 with the left eye. The patient had a comitant esotropia of 10 prism diopters and full extraocular 350 Galvez-Ruiz et al: J Neuro-Ophthalmol 2013; 33: 349-353 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. FIG. 1. The optic discs show temporal pallor in the 4 Saudi patients found to have OPA1 gene mutations. Galvez-Ruiz et al: J Neuro-Ophthalmol 2013; 33: 349-353 351 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. movements. Funduscopy revealed temporal pallor of both optic nerves (Fig. 1). KVF testing showed a cecocentral sco-toma in both eyes and an increase in the blind spot in the left eye. Brain and orbital MRI were normal. OPA1 sequencing detected a heterozygous A to T substitu-tion at position c.949-2A.T (IVS9-2A.T) in intron 9 of the OPA1 gene, affecting the invariant acceptor splice site consensus of exon 10. The novel splice site was subjected to different splice site prediction tools. The wild-type acceptor site was not recog-nized inNNSplice and Netgene2,which is not unusual especially for acceptor sites. However, the wild-type sequence obtained a score of 7.92 in MaxEnt (reference PMID: 15285897), whereas the mutant sequence was not recognized, supporting the assumption that the mutation should impair splicing. Case 4 A 35-year-old woman reported progressive visual loss over the past 10 years. She had a sister with decreased vision. There was no consanguinity between her parents. VA was 20/125, right eye, and 20/100, left eye. On the Ishihara test, she was able to distinguish 7 of 15 plates with the right eye and 5 of 15 plates with the left eye. Funduscopy revealed mild temporal pallor of both optic nerves (Fig. 1). KVF examination showed global loss of sensitivity in both eyes, and MRI evaluation was unremarkable. Analysis of OPA1 revealed the heterozygous mutation c.1313A.T, which results in a substitution of asparagine by valine at position 438 (p.Asp438Val). This missense mutation has been described previously (11) and likely results in func-tional null alleles. In none of the patients was a deletion or duplication in the OPA1 gene found. To the best of our knowledge, c.8S2T.A (Case 1) and C.949-2A.T (IVS9-2A.T) (Case 3) muta-tions of the OPA1 gene have not been reported previously. DISCUSSION To the best of our knowledge, this study presents the first DOA cases with OPA1 mutations in Saudi Arabia. Two patients had a nonsense mutation (1 of them with a novel mutation), 1 a missense mutation, and 1 had a novel splice site mutation. LHON testing was negative in all our patients, and none who were analyzed for alterations in OPA3 carried a muta-tion in this gene. It seems that OPA3 mutations are a rare cause of DOA. But we are cautious about this conclusion because MLPA analysis was not carried out and large-scale OPA3 gene rearrangements cannot be fully excluded. Our study is not the first attempt to identify OPA1 muta-tions in Saudi patients. In 2008, Bosley et al (12) published a report of patients from Saudi Arabia with sporadic bilateral optic neuropathy in infancy. The authors enrolled 21 patients and 159 control subjects and evaluated the evidence for mito-chondrial disease, including direct sequencing tests for OPA1 and OPA3. Their inclusion criteria consisted of bilateral decrease in VA since infancy, a lack of family history (i.e., only sporadic cases), and lack of clinical or any underlying etiologies that could explain optic atrophy. They failed to detect any cases with OPA1 or OPA3 genes. The authors found 1 patient who tested positive for LHON (11,778) and 3 additional patients with mutations that could be pathological for LHON. In contrast, we included 11 patients with a family history of optic nerve disease. However, of the 4 patients with a positive OPA1 test, all except 1 were sporadic. Therefore, it is unlikely that the lack of OPA1 mutations in the report by Bosley et al (12) was because of the inclusion of only sporadic patients. A study by Yu-Wai-Man et al (13) highlighted that having a family history increased the positivity of the OPA1/OPA3 test. In their series of 188 patients, the overall detection of OPA1 was 14.4%, but detection of OPA1 gene mutations was up to 50% among patients with a positive family history. Considering the high rates of consanguinity in Saudi Arabia, the low proportion of patients with a family history (27.5%) is surprising. We propose 3 possible explanations. First, given the high rate of consanguinity, a large proportion of patients with inherited optic nerve disease, especially the sporadic cases, were due to autosomal recessive transmission. Second, it was often difficult to establish the presence or lack of a family history. Many individuals were from remote regions of the country and were poorly informed about the causes of reduced vision in family members. Third, our hospital is a referral center for the entire country and some patients had to travel great distances. This made it difficult to examine and conduct genetic studies in family members. Regarding the presence of the DOA plus phenotype in our series, we found only 5 patients with hypoacusis and gait instability, and all of them had negative results for OPA1. This group represents 12.5% of patients with DOA in our series and is lower than in multicenter studies that have reported a preve-lance of 20% (13,14). This may be due to our small sample size or that the clinical manifestations of OPA1 mutations in Saudi Arabia differ from those detected elsewhere. Currently, several genetic screening or sequencing meth-ods, such as polymerase chain reaction and or single-strand conformational polymorphism analyses, are available (3,13). In addition, methods are available that can detect large-scale rearrangements, such as comparative genomic hybridization microarray technology and MLPA. One drawback of screen-ing methods is that they overlook large-scale rearrangements that could cause up to 20% of all OPA1 cases (3,13,15). In the study by Yu-Wai-Man et al (13), the application of meth-ods for detecting both point mutations and large-scale rear-rangements in a large series of probands with possible DOA did not increase the detection rate. The authors concluded that given the high cost of rearrangement detection techni-ques, these assays should be reserved for patients with a pos-itive family history. Yu-Wai-Man et al (13) recognized that false negatives cannot be completely excluded even when using rearrangement detection techniques. DOA has great genetic heterogeneity. To date, it has been associated with 5 chromosomal loci (OPA1, OPA3, 352 Galvez-Ruiz et al: J Neuro-Ophthalmol 2013; 33: 349-353 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. OPA4, OPA5, and OPA7), yet the causative genes have only been found for OPA1 and OPA3. It is likely that genetic alterations associated with different loci will be discovered in the future, including in the Saudi population. Not only is there extensive genetic heterogeneity of DOA but also large variability of its clinical expression (16-18). As genetic tests for OPA1 become more affordable, the reports of positive results in completely asymptomatic individuals have become increasingly common. For example, our Patient 1, who had a confirmed mutation in OPA1, did not exhibit any visual symptoms and came to our hospital seeking treatment for myopia. DOA was suspected only when mild bilateral optic disc pallor of the optic nerve was detected; as a result, a genetic test was requested, and the results were positive for DOA. REFERENCES 1. Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies-disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81-114. 2. Fraser JA, Biousse V, Newman NJ. The neuro-ophthalmology of mitochondrial disease. Surv Ophthalmol. 2010;55:299-334. 3. Yu-Wai-Man P, Griffiths PG, Hudson G, Chinnery PF. Inherited mitochondrial optic neuropathies. J Med Genet. 2009;46: 145-158. 4. Kamei S, Chen-Kuo-Chang M, Cazevieille C, Lenaers G, Olichon A, Belenguer P. Expression of the Opa1 mitochondrial protein in retinal ganglion cells: its downregulation causes aggregation of the mitochondrial network. Invest Ophthalmol Vis Sci. 2005;46:4288-4294. 5. Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008;131:352-367. 6. Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. II. Clinical and epidemiological aspects. Acta Ophthalmol Scand. 1996;74:3-7. 7. Hoyt CS. Autosomal dominant optic atrophy-a spectrum of disability. Ophthalmology. 1980;87:245-251. 8. Votruba M, Fitzke FW, Holder GE, Carter A, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol. 1998;116:351-358. 9. Spinazzola A, Invernizzi F, Carrara F, Lamantea E, Donati A, Dirocco M. Clinical and molecular features of mitochondrial DNA depletion syndromes. J Inher Metab Dis. 2009;32:143-158. 10. Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. 11. Pesch UE, Leo-Kottler B, Mayer S, Jurklies B, Kellner U, Apfelstedt-Sylla E, Zrenner E, Alexander C, Wissinger B. OPA1 mutations in patients with autosomal dominant optic atrophy and evidence for semi-dominant inheritance. Hum Mol Genet. 2001;10:1359-1368. 12. Bosley TM, Brodsky MC, Glasier CM, Abu-Amero KK. Sporadic bilateral optic neuropathy in children: the role of mitochondrial abnormalities. Invest Ophthalmol Vis Sci. 2008;49:5250-5256. 13. Yu-Wai-Man P, Shankar SP, Biousse V, Miller NR, Bean LJ, Coffee B, Hegde M, Newman NJ. Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology. 2011;118:558-563. 14. Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W Jr, Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF. Multi-system neurological disease is common in patients with OPA1 mutations. Brain. 2010;133:771-786. 15. Ranieri M, Del Bo R, Bordoni A, Ronchi D, Colombo I, Riboldi G, Cosi A, Servida M, Magri F, Moggio M, Bresolin N, Comi GP, Corti S. Optic atrophy plus phenotype due to mutations in the OPA1 gene: two more Italian families. J Neurol Sci. 2012;315:146-149. 16. Cohn AC, Toomes C, Potter C, Towns KV, Hewitt AW, Inglehearn CF, Craig JE, Mackey DA. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007;143:656-662. 17. Toomes C, Marchbank NJ, Mackey DA, Craig JE, Newbury- Ecob RA, Bennett CP, Vize CJ, Desai SP, Black GCM, Patel N, Teimory M, Markham AF, Inglehearn CF, Churchill AJ. Spectrum, frequency and penetrance of OPA1 mutations in dominant optic atrophy. Hum Mol Genet. 2001;10:1369-1378. 18. Thiselton DL, Alexander C, Taanman JW, Brooks S, Rosenberg T, Eiberg H, Andreasson S, Van Regemorter N, Munier FL, Moore AT, Bhattacharya SS, Votruba M. A comprehensive survey of mutations in the OPA1 gene in patients with autosomal dominant optic atrophy. Invest Ophthalmol Vis Sci. 2002;43:1715-1724. Galvez-Ruiz et al: J Neuro-Ophthalmol 2013; 33: 349-353 353 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |