| OCR Text |
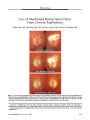
Show ORIGINAL CONTRIBUTION Pupillographic Findings in 39 Consecutive Cases of Harlequin Syndrome Fion Bremner, PhD, FRCOphth and Stephen Smith, MD, PhD Background: Harlequin syndrome is a curious phe-nomenon in which one half of the face fails to flush during thermal or emotional stress as a result of damage to vasodilator sympathetic fibers. Anecdotal reports suggest that some of these patients have abnormal pupils. In this study we set out to system-atically investigate autonomic pupil disturbances in an unselected cohort of patients with harlequin syndrome. Methods: A consecutive series of 39 patients with harlequin syndrome who were referred to a tertiary autonomic function laboratory underwent slit-lamp examinations, testing of deep tendon reflexes, in-frared video pupillography and, where needed, addi-tional pharmacologic pupillary testing. Results were compared with a meta-analysis of all previously reported cases of harlequin syndrome (n = 39) identified from a literature search. Results: In 65% of patients, no underlying causative medical disturbance could be identified. In 64% of patients, there were abnormal pupils, most commonly Horner syndrome, which was always present ipsilat-eral to the side of the face with impaired facial sweating and flushing. The lesion was postganglionic in 9 of 10 patients tested pharmacologically. Five (13%) patients had tonic pupils, most of whom also had tendon areflexia but no other neurologic findings, a pattern consistent with Holmes-Adie syndrome. In 2 of these patients, tonic and Horner pupils coexisted. Normal pupils were present in 36% of patients. These results are similar to those for the 39 previously reported patients with harlequin syndrome. Conclusions: The frequent coexistence of harlequin and Horner syndromes without other neurologic deficits suggests pathologic changes affecting the superior cervical ganglion. Because either syndrome may occur alone, damage is apparently selective. Among the patients with harlequin syndrome who also have tonic pupils and tendon areflexia (Holmes- Adie syndrome), we postulate a ganglionopathy affecting not merely the (sympathetic) superior cervical ganglion, but also the (parasympathetic) ciliary and dorsal root ganglia. Because we found that more than 10% of patients had an undisclosed mass lesion in the chest or neck or a generalized autonomic neuropathy, we recommend a targeted evaluation in selected patients with harlequin syndrome. (J Neuro-Ophthalmol 2008;28:171-177) In his original article, Horner (1) described reduced sweating above the eyebrow in patients with oculosym-pathetic paresis. Compared with sweating, the vasomotor control of cutaneous blood flow in the face is more com-plicated and is subject to a number of sophisticated control systems (2), of which sympathetically mediated active vasodilatation is only one (Fig. 1). In 1988, Lance et al (3) reported a series of 5 patients in whom there was sudden loss of facial flushing on one side of the face after heat stress or exercise, resulting in a striking demarcation line between the reddened contralateral side of the face and the pale ipsilateral side of the face. They called this condition the ‘‘harlequin syndrome.'' The authors demonstrated in 4 of these 5 patients that ipsilateral gustatory flushing/sweating was preserved and proposed that harlequin syndrome was caused by damage to the preganglionic sympathetic path-way. However, only 1 of their patients had Horner syn-drome, implying that the lesion in the remaining 4 patients lay distal to the point of exit of the sympathetic pupillo-motor fibers from the spinal cord, at level T2-T3 rather than T1. There have since been a number of reports of harlequin syndrome [reviewed by Wasner et al (4)], and in some cases the patients also had a tonic pupil similar to that seen in the Holmes-Adie syndrome (5-8), implying concomitant damage to the postganglionic parasympathetic pupillomotor fibers. These reports have suggested that there is a nosologic relationship between Holmes-Adie syndrome Department of Neuro-Ophthalmology, National Hospital for Neurol-ogy and Neurosurgery, London, United Kingdom. Address correspondence to Fion D. Bremner, PhD, FRCOphth, Consultant Ophthalmic Surgeon, Department of Neuro-Ophthalmology, Internal Box 142, National Hospital for Neurology and Neurosurgery, London WC1N 3BG, United Kingdom; E-mail: fion.bremner@uclh.nhs.uk J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 171 (HAS), which includes tonic pupils and tendon areflexia, Ross syndrome [HAS with patchy hypohidrosis (9)] , and harlequin syndrome (7) and that both the parasympathetic and the sympathetic pathways may be affected in these conditions. Alternatively, the concurrence of these con-ditions may be coincidental. The published literature on this topic is restricted to anecdotal case reports from which it is impossible to assess the frequency of concurrence of these different syndromes. We report here the pupillographic findings in an unselected consecutive series of 39 cases of harlequin syndrome and compare our results with those in 39 previously reported cases. METHODS Subjects We examined 39 consecutive patients with harlequin syndrome referred for autonomic function or pupillographic tests. There were 15 men and 24 women with a median age of 46 years (range, 10-74 years). The diagnosis of harlequin syndromewas confirmed by clinical observation of unilateral flushing after exercise-induced heat stress. Pupillography Slit-lamp examination of the iris, pupil, and anterior segment of the eye was performed. Pupil diameters and their responses to a bright flash of light (duration, 1.0 FIG. 1. Mechanisms for active cutaneous vasodilation in the face. Heat (thermal stress) or embarrassment (emotional stress) cause facial flushing/blushing (and sweating) by activation of preganglionic sympathetic neurons [Symp] that exit the spinal cord at T2/T3 and terminate in the superior cervical ganglion. The postganglionic fibers are distributed to the forehead via the internal carotid plexus and to the rest of the face via branches of the external carotid artery. It is not known whether sympathetic vasodilation is mediated directly by vasomotor fibers or indirectly via cholinergic sudomotor fibers releasing vasoactive substances from sweat glands. Active parasympathetic [P/S] vasodilation (and lacrimation) results from irritation of the eyes or nose and is mediated via a trigeminofacial reflex and the superior salivatory nucleus (SSN). The preganglionic efferents terminate in the sphenopalatine ganglion and postganglionic fibers then cause vasodilation probably by release of vasoactive intestinal polypeptide (VIP) or nitric oxide (NO). Noxious stimuli in the face bring about vasodilation by local axon reflexes causing antidromic release of substance P or calcitonin gene-related product (CGRP). V, fifth cranial nerve. 172 q 2008 Lippincott Williams & Wilkins J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 Bremner and Smith second) and an accommodative effort (‘‘near response'') were recorded in all but 1 patient by infrared video pupillometry. The pupil measurements were compared with a large normative database obtained from healthy age-matched control subjects. Sympathetic or parasympathetic deficits were diagnosed according to strict criteria as described in an earlier publication (10). One patient, a 33- year-old woman who was quadriplegic from Guillain-Barre´ syndrome with autonomic involvement, was too ill to undergo formal pupillography; her pupils were examined clinically at the bedside and photographed before and after eyedrop administration. Horner syndrome was diagnosed on the basis of redilatation lag [abnormally prolonged time for 75% recovery to baseline, T3/4, as defined in our previous publication (10)]. In patients with pupillotonia (which itself delays redilatation), the diagnosis of Horner syndrome rested on failure of pupils to dilate in response to cocaine. Tonic pupils were diagnosed on the basis of attenuation of the light response, a slow but exaggerated near response with light-near dissociation, by sector palsy, and in 2 patients, by denervation supersensitivity to topical 0.1% pilocarpine (10). Pharmacologic Studies Pharmacologic testing was used in a small number of patients in whom the diagnosis of a sympathetic or para-sympathetic deficit was still unclear after standard pupillo-graphic examination, in the patient too ill to undergo formal pupillography, and in patients in whom localization of the lesion causing Horner syndrome was needed. Tests of sympathetic integrity included topical 4% cocaine, 1% phenylephrine, or 1% hydroxyamphetamine. Dilute 0.1% pilocarpine was used to test for parasympathetic supersensitivity. Deep Tendon Reflexes Supinator, biceps, triceps, patellar, and Achilles tendon reflexes were examined in 30 patients by routine clinical testing. Statistics Differences between continuous variables were com-pared with Student's t test and categorical differences by x 2 tests. RESULTS Underlying Medical Conditions The medical diagnoses reached after investigation are shown in Table 1. In 10 (23%) patients, there were one or more causally related medical conditions: 2 brachial plexopathies caused at birth by forceps delivery, 2 pure autonomic failure (PAF), 1 multiple system atrophy (MSA), 1 Guillain-Barre´ syndrome, 1 unspecified dysautonomia, 1 apical lung (Pancoast) tumor (poorly differentiated adenocarcinoma treated by surgery and radiotherapy 4 years earlier), 1 thoracic sympathectomy, and 1 type 1 diabetes (but with no evidence of autonomic neuropathy elsewhere). In the patient with MSA and in 1 of the 2 patients with PAF, the harlequin syndrome antedated the general autonomic failure by years. In 5 (12%) patients, there were conditions only possibly causally related, including 1 patient with each of the following: discoid lupus erythematosus, trigeminal neuralgia, idiopathic bladder dysfunction associated with an unidentified lower spinal cord lesion, previous axillary surgery for hyperhidrosis, and repeated vasovagal attacks. In these last 2 patients, no definite abnormalities were found in a comprehensive battery of autonomic function tests. In 24 (65%) patients, no causally-related medical conditions were apparent. Flushing and Sweating The right side was affected in 17 patients and the left in 22. The extent of the phenomenon varied widely. In 22 patients only the face was involved; in 3 patients the neck was involved, and in the remaining 14 patients the trunk was involved, with or without effects in the arm or leg. Figure 2 shows an example of one of these patients. After exercise, the left side of his face failed to vasodilate (flush) or sweat in contrast with the normal right side, giving rise to a striking harlequin appearance. Slit-Lamp Findings Visible abnormalities of the pupil or iris when exam-ined at the slit-lamp were found in 6 (15%) patients, including heterochromia iridis in the 2 patients with birth injuries, sector palsies in 2 patients with bilateral pupillotonia, and irregularly-shaped pupils without definite sector palsy in 2 patients with unilateral pupillotonia. In the remaining 33 patients, the pupils looked normal. Pupillographic Findings Twenty-five (64%) patients had abnormal pupils when examined pupillographically (Table 2). The most common abnormality was ipsilateral Horner syndrome, found in 18 (46%) patients. In 11 of these patients, this was the only abnormality. Three patients had additional para-sympathetic deficits and 4 had bilateral Horner syndrome. Topical 1% hydroxyamphetamine testing, performed in 10 of the patients with a unilateral Horner syndrome (8 isolated and 2 with tonic pupils), disclosed a postganglionic lesion in 9 (90%). The only patient with a preganglionic lesion was the man with an apical lung (Pancoast) tumor. Tonic pupils were found in 5 (13%) patients. The pupillotonia was bilateral in 2 and unilateral in 3 173 Pupils in Harlequin Syndrome J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 (1 ipsilateral and 2 contralateral). In 1 of these patients, the diagnosis of tonic pupils had been made 25 years earlier. In the other patients, onset could not be determined as the pupillotonia was asymptomatic and had not been noticed by the patients or their referring clinicians. Supersensitivity to 0.1% pilocarpine was found in both of the patients in which this test was performed. Evidence for combined parasympathetic and sympa-thetic pupillary deficits was found in 3 (7%) patients. An example is shown in Figure 3. This was a 63-year-old woman with ipsilateral Horner syndrome and a tonic pupil in the contralateral eye but with no evidence of a generalized dysautonomia. Of the 2 additional patients with combined parasympathetic and sympathetic deficits, one had the same pattern as shown in Figure 3, and the other had bilateral pupillotonia with an ipsilateral Horner syndrome. Five (13%) patients had pupils that, although not definitely tonic or typical of Horner syndrome, were clearly abnormal. One of these patients, a 46-year-old woman, had an abnormal degree of anisocoria in the dark, the larger pupil being ipsilateral to the harlequin syndrome. This larger pupil showed no redilatation lag but had a relatively poor mydriatic response to topical cocaine compared with that for the other eye. A second patient, a 53-year-old woman, had equal but abnormally large pupils for her age on both sides; the pupil on the harlequin side also showed borderline redilatation times. A third patient, a 48-year-old woman, had abnormally small pupillary light responses without evidence of afferent visual pathway disease yet normal-amplitude brisk pupil constriction when viewing a near target. The 2 remaining patients, women aged 28 and 64, had abnormally large anisocoria both in darkness and in light. In both patients, the smaller pupil was on the same side as the harlequin syndrome, and light reflexes were attenuated with intact, brisk near responses. Pharmacologic testing in both of these patients showed symmetrically normal mydriatic responses to 4% cocaine but greater than expected miotic responses to 0.1% pilocarpine. There was no pupillographic or pharmacologic evi-dence of either sympathetic or parasympathetic pupillary deficits in 14 (36%) patients with harlequin syndrome. Correlation of Pupil Findings With Other Neurologic Observations No relationship was found between the presence or absence of pupil abnormalities and the extent of the area affected by the harlequin syndrome. Nine patients had asymmetric or absent tendon jerks (Table 1), including the TABLE 1. Underlying medical diagnoses, pupil findings, and tendon jerks in 39 patients with harlequin syndrome No. cases Pupil findings Tendon jerks I. Definite causally-related medical condition (n = 10) Birth injury 2 Hu (2) N Pure autonomic failure 2 Hb (2) N Multiple system atrophy 1 Hu N Guillain-Barre´ syndrome 1 Hb A Idiopathic dysautonomia 1 Tb A Pancoast tumor 1 Hu N Thoracic sympathectomy 1 N N Diabetes 1 N N II. Possible causally-related medical condition (n = 5) Discoid lupus 1 Tb/Hu A Trigeminal neuralgia 1 Hu N Bladder dysfunction 1 N A Hyperhidrosis 1 A N Vasovagal attacks 1 Tu A III. No causally-related medical condition (n = 24) 24 Hu (6) A(1); N(5) Hb (1) N Tu (1) A Tu/Hu (1) A A (4) N N (11) A(1); N(10) N, normal; A, abnormal; Hb, bilateral Horner syndrome; Hu, unilateral Horner syndrome; Tb, bilateral pupillotonia; Tu, unilateral pupillotonia. 174 q 2008 Lippincott Williams & Wilkins J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 Bremner and Smith patient with Guillain-Barre´ syndrome, all 5 patients who had at least one tonic pupil, 1 patient who had unilateral Horner syndrome, and 2 patients with normal pupils. Standard cardiovascular and thermoregulatory autonomic function tests showed widespread abnormalities in the 5 patients known to have generalized dysautonomia, but results were normal in all other patients with harlequin syndrome. DISCUSSION Our results are in general agreement with those in 39 previously reported cases of harlequin syndrome (3-8,11- 28) (Table 3). Women seem to be more commonly affected than men, perhaps reflecting a greater propensity among women to seek advice for embarrassing asymmetry of facial flushing rather than any particular susceptibility of their sympathetic vasodilator fibers to injury. The median age at presentation was similar for men (47 years) and women (45 years) in our study and was consonant with the median presenting age (43 years) for women in the published literature. The median presenting age for men among published cases was significantly lower (21 years) than ours, perhaps owing to a disproportionate number of published cases in the pediatric literature. Our study also agrees with the published literature in finding that the majority of patients with harlequin syn-drome have abnormal pupils. The most common pupil abnormality is Horner syndrome, which was found in 46% of our patients and in 38% of previously reported patients. This finding is not surprising in view of the emerging evidence regarding the neural basis for harlequin syndrome. An active sympathetic vasodilator mechanism in the face has been demonstrated by Drummond and Finch (29) in 10 patients subjected to heat stress after stellate ganglion blockade. Most of these sympathetic vasodilator fibers leave the spinal cord at the level of T2-T3 (30) and travel up the sympathetic chain to terminate in the superior cervical ganglion. Postganglionic fibers are then distributed to the vascular beds of the upper and lower face via branches of TABLE 2. Distribution of pupil findings in 34 patients with harlequin syndrome Parasympathetic deficit Sympathetic deficit None Unilateral deficit Bilateral deficit None 14 11 4 Unilateral deficit 1 2 0 Bilateral deficit 1 1 0 Five patients were excluded from this analysis because although their pupils were abnormal, a definite diagnosis of sympathetic or parasympathetic deficit could not be made. FIG. 3. Pupillary responses to a 1-second flash of light in a 63-year-old woman with left harlequin syndrome. The left pupil (L) shows a redilatation lag due to an ipsilateral Horner syndrome. The right pupil (R) shows no response to light due to pupillotonia. FIG. 2. Harlequin syndrome. A 56-year-old man shows post-exercise flushing and sweating restricted to the right half of the face. The pupils are normal. 175 Pupils in Harlequin Syndrome J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 the internal and external carotid arteries, respectively. For much of their journey, these sympathetic vasodilator fibers travel alongside the sympathetic pupillomotor fibers and share susceptibility to damage from lesions in the chest and neck. The lesion of harlequin syndrome has been localized in very few of the published reports, but we found that 9 of 10 patients with harlequin and Horner syndromes had postganglionic sympathetic pathway lesions. In all of these cases, the patients were otherwise healthy, and extensive investigations revealed no cause for the harlequin or Horner syndrome. In the absence of any evidence for a diffuse autonomic neuropathy, we assume these patients had focal lesions of the superior cervical ganglion. Some 15% of our patients and some 26% of reported patients with harlequin syndrome cases had tonic pupils. The onset of the pupillary signs sometimes preceded the harlequin syndrome by as much as 25 years, although in most patients the precise temporal relationship cannot be established. Many of these patients with tonic pupils also had tendon areflexia and no other neurologic findings, suggesting a diagnosis of HAS. It is known that some patients with HAS also have evidence of patchy sudomotor dysfunction (9), so it seems that sympathetic ganglia can be affected as well as the dorsal root and other parasym-pathetic ganglia, raising the possibility that the concurrence of HAS and harlequin syndrome may be more than just coincidence. Thompson (31) estimated the prevalence of HAS in the population of Iowa at only 2 per 1,000. If a similar prevalence is assumed for the populations from which these patients with harlequin syndrome are drawn, then the rate at which we have observed both conditions together (equivalent to 206 per 1,000) is more than 100 times greater than expected by chance alone (x2 = 165.8; P < 0.001 using Yates correction for continuity). Thus, it is likely that the etiologic agent responsible for HAS can also cause harlequin syndrome years or decades later in some patients. Given that the manifestations of HAS are associated with damage to ganglia, the site of damage in harlequin syndrome is likely to be the superior cervical ganglion. Such lesions may present as vasomotor (harlequin), sudomotor (Ross), or pupillomotor (postganglionic Horner) dysfunc-tion, depending on the sensitivity of the tests used to examine the patient and other unknown factors. In 5 of 39 patients in our series, the pupil examination was abnormal but did not meet our diagnostic criteria for a tonic pupil or Horner syndrome. It is likely that some of our pupil measurements lay outside the 95% normal limits by chance alone, but the relatively high frequency of observing these borderline cases and the findings of several variables outside the normal limits in each patient imply that in some patients there is a mild disturbance in autonomic innervation of the pupil.We intend to reexamine these patients after a few years to see if their pupils will become more convincingly tonic or show a definite Horner syndrome in the future. In one third of our patients and patients in published reports, results of the pupil examination were entirely normal. It is possible that Horner syndrome was missed in some of these patients, as neither redilatation times nor pharmacologic tests have 100% sensitivity in detecting an oculosympathetic deficit. But such ‘‘missed'' diagnoses probably account for only a few patients labeled as having normal pupils. In instances of ‘‘isolated'' harlequin syn-drome without other evidence of autonomic dysfunction, we must conclude that the lesion is small or the damage is selective to certain fiber populations within the sympathetic pathway. In our series, all 5 patients with generalized dysautonomia had abnormal pupils, although in 1 patient the deficits were parasympathetic rather than sympathetic. The rarity of diagnosing parasympathetic and sympathetic deficits in these patients may indicate the fact that autonomic disturbances are often patchy rather than diffuse in their distri-bution ormay simply reflect the technical difficulty in unmask-ing a mild sympathetic deficit in the face of overwhelming parasympathetic denervation of the pupil (9,32). Harlequin syndrome has previously been regarded as a curious but generally benign neurologic condition rarely associated with a serious underlying pathologic lesion. TABLE 3. Meta-analysis of pupil findings and underlying medical diagnoses in 39 previous reported cases of harlequin syndrome Pupil diagnoses No. cases Underlying medical diagnoses References Horner 15 Anesthesia (12,13), paravertebral tumor (15), mass in neck (4,18), congenital (19) 3, 4, 12-20 Tonic 3 Multiple sclerosis (11) 7, 8, 11 Horner 1 tonic 6 5-7 Normal 10 Central line (21), neck surgery (24) 3, 15, 21-24 Not stated/not clear 5 Parasomnia (26), anesthesia (27), mediastinal neurinoma (28) 25-28 176 q 2008 Lippincott Williams & Wilkins J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 Bremner and Smith Indeed, the overwhelming majority of patients in our series and in the published literature had no other neurologic or health problems. However, 3 of the patients in the published reports and 1 patient in our series had mass lesions in the neck or mediastinum. Furthermore, 5 of our patients had generalized dysautonomia, a high prevalence that may reflect the pattern of referrals to our institution, where we have a particular interest in these conditions. Of these patients with dysautonomia, 3 presented with harlequin syndrome before any other manifestations of an underlying neurologic disease had become apparent. Given this find-ing, it may be advisable in some patients with clinically isolated harlequin syndrome to arrange more detailed investigations of their autonomic and peripheral nervous systems or at least to keep them under surveillance. Acknowledgments I am deeply indebted to my co-author Stephen Smith, who had a great interest in these patients. He died last year before the conclusion of this study, but it was his enduring wish that I publish this case material. The pupillometry equipment was built with a generous grant from Fight for Sight. REFERENCES 1. Horner JF. Ueber eine Form von Ptosis. Klin Monatsbl Augenh 1869; 7:193-8. 2. Drummond PD. Sweating and vascular responses in the face: normal regulation and dysfunction in migraine, cluster headache and harlequin syndrome. Clin Auton Res 1994;4:273-85. 3. Lance JW, Drummond PD, Gandevia SC et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psychiatry 1988;51:635-42. 4. Wasner G, Maag R, Ludwig J et al. Harlequin syndrome-one face of many etiologies. Nat Clin Pract Neurol 2005;1:54-9. 5. Drummond PD, Edis RH. Loss of facial sweating and flushing Holmes-Adie syndrome. Neurology 1990;40:847-9. 6. Caparros-Lefebrve D, Hache JC, Hurtevent JF et al. Unilateral loss of facial flushing and sweating with contralateral anhidrosis: harlequin syndrome or Adie's syndrome? Clin Auton Res 1993;3:239-41. 7. Shin RK, Galetta SL, Ting TY et al. Ross syndrome plus, beyond Horner, Holmes-Adie harlequin. Neurology 2000;55:1841-6. 8. Kalapesi FB, Krishnan AV, Kiernan MC. Segmental facial anhidrosis and tonic pupils with preserved deep tendon reflexes: a novel autonomic neuropathy. J Neuroophthalmol 2005;25:5-8. 9. Ross AT. Progressive selective sudomotor degeneration: a case with coexisting Adie's syndrome. Neurology 1958;8:809-17. 10. Bremner F, Smith S. Pupil findings in a consecutive series of 150 patients with generalised autonomic neuropathy. J Neurol Neurosurg Psychiatry 2006;77:1163-8. 11. Carroll CB, Zajicek JP. The harlequin' sign in association with multiple sclerosis. J Neurol 2004;251:1145-6. 12. Burlacu CL, Buggy DJ. Coexisting harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth 2005;95: 822-4. 13. Crawley SM. Coexisting harlequin and Horner syndromes after high thoracic paravertebral block. Br J Anaesth 2006;96:537-8. 14. Drummond PD, Lance JW. Site of autonomic deficit in Harlequin syndrome: local autonomic failure affecting the arm and the face. Ann Neurol 1993;34:814-9. 15. ten Holter JB, Visser A. Harlequin syndrome. Ned Tijdschr Geneeskd 1997;141:2495-9. 16. Montigiani A, Cencetti S, Bandinelli G et al. The harlequin sign': case description and review of the literature. Arch Ital Med Int 1998; 13:173-5. 17. Morrison DA, Bibby K, Woodruff G. The harlequin' sign and congenital Horner's syndrome. J Neurol Neurosurg Psychiatry 1997; 62:626-8. 18. Padda GS, Cruz OA, Silen ML et al. Skin conductance responses in paediatric harlequin syndrome. Paediatr Anaesth 1999;9:159-62. 19. Saito H. Congenital Horner's syndrome with unilateral facial flushing. J Neurol Neurosurg Psychiatry 1990;53:85-6. 20. Swan MC, Nicolaou M, Paes TR. Iatrogenic harlequin syndrome. Postgrad Med J 2003;79:278. 21. Coleman PJ, Goddard JM. Harlequin syndrome following internal jugular vein catheterisation in an adult under general anesthesia. Anesthesiology 2002;97:1041. 22. Fallon KE, May JJ. Harlequin syndrome in two athletes. Br J Sports Med 2005;39:e1. 23. Moon SY, Shin DI, Park SH et al. Harlequin syndrome with crossed sympathetic deficit of the face and arm. J Korean Med Sci. 2005;20: 329-30. 24. Turco GR, Fraber NE. Postoperative autonomic deficit: a case of harlequin syndrome. Anesthesiology 1996;85:1197-9. 25. Corbett M, Abernethy DA. Harlequin syndrome. J Neurol Neurosurg Psychiatry 1999;66:544. 26. Lombardi C, Vetrugno R, Provini F et al. Harlequin syndrome: an association with overlap parasomnia. J Neurol Neurosurg Psychiatry 2004;75:341-2. 27. Mashour GA, Levine W, Ortiz VE. Intraoperative harlequin syndrome. Anesth Analg 2006;102:655. 28. Noda S. Harlequin syndrome due to mediastinal neurinoma. J Neurol Neurosurg Psychiatry 1991;54:744. 29. Drummond PD, Finch PM. Reflex control of facial flushing during body heating in man. Brain 1989;112:1351-8. 30. Nathan PW, Smith MC. The location of descending fibres to sympathetic neurons supplying the eye and sudomotor neurons supplying the head and neck. J Neurol Neurosurg Psychiatry 1986; 49:187-94. 31. Thompson HS. Adie's syndrome: some new observations. Trans Am Ophthalmol Soc 1977;75:587-626. 32. Bremner FD, Smith SE. Pupil abnormalities in selected autonomic neuropathies. J Neuroophthalmol 2006;26:209-19. 177 Pupils in Harlequin Syndrome J Neuro-Ophthalmol, Vol. 28, No. 3, 2008 |