| OCR Text |
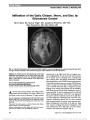
Show Retinal Nerve Fiber Layer Thickness, Brain Atrophy, and Disability in Multiple Sclerosis Patients Jose Manuel Abalo-Lojo, MD, Carmen Carollo Limeres, MD, Manuel Arias Gómez, MD, Sandra Baleato-González, MD, Carmen Cadarso-Suárez, MD, Carmen Capeáns- Tomé, MD, Francisco Gonzalez, MD Objective: To study the relationship between retinal nerve fiber layer (RNFL) thickness and brain atrophy using magnetic resonance imaging (MRI) with bicaudate ratio (BCR) in patients with multiple sclerosis (MS) with different levels of disease severity. We also assessed whether RNFL thickness correlated with Expanded Disability Status Scale (EDSS) score. Methods: The participants consisted of 88 patients with MS and 59 age- and sex-matched healthy control subjects. Eleven patients had clinically isolated syndrome (CIS), 68 patients had relapsing-remitting MS (RR-MS), and 9 patients had secondary progressive MS. Patients and controls were evaluated using optical coherence tomography (OCT, Cirrus) and scanning laser polarimetry with variable corneal compen-sation (GDx VCC). Patients underwent the same brain MRI scanning protocol. Disability was evaluated according to the EDSS. The BCR was calculated by dividing the minimum intercaudate distance by brain width along the same level. Results: The BCR was higher in patients with MS (0.12 ± 0.03) than in controls (0.08 ± 0.009) (P , 0.001). OCT average RNFL thickness in patients with MS was signifi-cantly lower (84.51 ± 14.27 mm) than in control subjects (98.44 ± 6.83 mm). BCR was correlated with OCT average RNFL thickness (r = 20.48, P = 0.002) in patients with MS without optic neuritis. Significant correlations were found between average RNFL thickness and EDSS (r = 20.43, P = 0.003). Additionally, there were correlations between BCR with GDx parameters in patients with MS without optic neuritis. Conclusions: This study shows that RNFL thickness corre-lates with BCR and with MS subtypes. Additionally, our study indicates that OCT is better suited for MS assess-ment than GDx. We conclude that the damage of retinal axons appears related to brain damage in patients with MS. Journal of Neuro-Ophthalmology 2014;34:23-28 doi: 10.1097/WNO.0000000000000057 © 2013 by North American Neuro-Ophthalmology Society Multiple sclerosis (MS) is a degenerative disorder of the central nervous system characterized by areas of demy-elination and axonal injury. Even at early stages, brain atrophy can be detected histopathologically and with magnetic reso-nance imaging (MRI). Although MS historically has been considered a white matter disease, many studies have dem-onstrated prominent changes in gray matter as well (1). The prechiasmal anterior visual pathways provide an attractive model for assessing the relationships between inflammation, demyelination, and neurodegeneration in MS. Axons emanating from retinal ganglion cells first display the morphologic characteristics of nonmyelinated (gray matter) fibers within the retinal nerve fiber layer (RNFL), and then become myelinated within the optic nerve, where they form a white matter tract (2). Several studies have estab-lished the presence of RNFL atrophy and reduction in mac-ular thickness in patients with MS (3-6). Optical coherence tomography (OCT) and scanning laser polarimetry are proven techniques to measure RNFL thickness and provide a method to measure visual pathway axonal injury. MRI is well suited to assess alteration in brain structure, including brain atrophy, in patients with MS (7). Brain Service of Ophthalmology (JMA-L, CC-T, FG), Complexo Hospital-ario Universitario de Santiago de Compostela, La Coruña, Spain; Department of Statistics (CCL, CC-S), School of Medicine, University of Santiago de Compostela, Spain; Service of Neurology (MAG), and Radiology (SBG), Complexo Hospitalario Universitario de San-tiago de Compostela, Spain; Department of Surgery and CIMUS (FG), University of Santiago de Compostela, Spain; and Insituto de Investigacion Sanitaria (FG), Santiago de Compostela, Spain. Supported by MCINN, Spain (BFU-2010-14968); Xunta de Galicia, Spain (10PXIB208126PR); and Ministerio de Industria y Com-petitividad (MTM2011-28285-C02-01). The authors report no conflicts of interest. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com). The data of this work are part of a doctoral thesis published in http:// hdl.handle.net/10347/5079. Address correspondence to Jose M. Abalo-Lojo, MD, Service of Ophthalmology, Complexo Hospitalario Universitario de Santiago de Compostela, E-15706 Santiago de Compostela, Spain; E-mail: jmabalolojo@yahoo.es Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 23 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. atrophy may occur early in the course of MS and may be associated with disability. The bicaudate ratio (BCR) is a method using MRI for estimating brain atrophy in normal aging (8,9) and in patients with MS (10). The BCR is calculated by dividing the minimum intercaudate distance by brain width along the same level, and reflects subcortical atrophy. It has been shown that BCR is correlated with gray and white matter atrophy in patients with MS (11) and used to monitor progression in patients with MS (12). The aim of our study was to investigate the relationship between RNFL thickness and brain atrophy in patients with MS using the BCR. We also studied whether RNFL thickness correlated with the Expanded Disability Status Scale (EDSS) (13). PATIENTS AND METHODS Patients This case-control study consisted of 88 patients (62 women and 26 men; mean age 39.19 ± 9.62 years and 39.85 ± 8.00 years, respectively) referred to the Ophthalmology Service from neurology of the Complexo Hospitalario Uni-versitario de Santiago de Compostela between January 2010 and May 2011. The study received approval of the local ethics committee. Eleven patients presented with clinically isolated syndrome (CIS), 68 patients had relapsing-remitting MS (RR-MS), and 9 patients had secondary progressive MS (SP-MS). MS was diagnosed according to Poser criteria (14). For the RR-MS subtype, we further subdivided the patients into benign MS and nonbenign MS. Patients with benign MS had a score on the EDSS of #3.0 at least 10 years after the onset of disease (15). A history of optic neuritis (ON) episodes was determined for eyes of patients with MS by self-report and physician report, and was confirmed by medical record review. We included patients with ON, but only if the episode occurred more than 6 months preceding enrollment in this study. A total of 43 patients had a history of ON, which was bilateral in 10 cases and unilateral in 33 cases. Fifty-nine age- and sex-matched healthy controls with no history of ocular or neurological disease were recruited. All had visual acuity of 20/20 in each eye. Patients with comorbid ocular conditions not related to MS or other causes of optic atrophy, such as glaucoma, ischemic optic neuropathy, or compressive optic neuropa-thy also were excluded. Patients with acute optic neuritis (within the 6 months before the study) were also excluded. None of the patients with MS had any other major medical illness or pre-existing medical conditions known to be associated with brain pathology. OCT was performed on both eyes using a Cirrus HD-OCT (Model 4000, Carl Zeiss, Meditec, Inc, Dublin, CA). The RNFL measurements were made using the Optic Disc Cube 200 · 200 protocol (200 horizontal scan lines, each composed of 200 A-scans) to generate a 6-mm square grid. Good-quality OCT scans were defined by a signal strength of 7 or greater (maximum, 10) and uniform brightness across the scan circumference. All scans were carried out by the same technician. The average thickness of the RNFL was used for statistical analysis. The GDx VCC (Laser Diagnostic Technologies, San Diego, CA) confocal scanning laser ophthalmoscope also was used to determine RNFL thickness. Circular scans (3.2 mm in diameter) centered on the optic disk were obtained. Pupils were dilated when image acquisition was impaired by small pupil size. Scan quality was considered adequate when the quality number was 7 or greater. The nerve fiber index (NFI) and temporal-superior-nasal-inferior-temporal (TSNIT) average RNFL thickness obtained with the GDx were used for statistical analysis. The NFI provides a single number (range, 1-100) representing the overall integrity of the RNFL. High NFI values indicate thinner RNFL. NFI values of #30 are considered normal. Patients underwent the same brain MRI scanning protocol at baseline using a 1.5T MRI scanner (Magneton Vision; Siemens, Erlangen, Germany). BCR was measured from a FLAIR axial image where the heads of the caudate nuclei were most visible and closest to each other (Fig. 1). We did not have access to the MRI in 5 patients with MS, and the BCR was not assessed in those cases. FIG. 1. Bicaudate ratio (BCR) is determined by the minimal intercaudate distance (d, arrows with solid line) and the distance between the outermost part of both hemispheres (D, arrows with broken line) measured at the same level. The BCR is the calculated by dividing the first and second measurement (d/D). 24 Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Disability was evaluated according to the EDSS score, which range from 0 to 10 with higher scores indicating more severe disability. Physical disability was assessed by a single experienced neurologist blinded to the MRI findings by using the EDSS within 1 week of the MRI. Disease duration was defined from the time of the first manifestation of disease until trial enrollment. Statistical Analysis Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS). Descriptive statistics and plots were created to determine whether the continuous variables (RNFL, NFI, TSNIT, and BCR) were normally distributed. All variables fit a normal distribution, except for NFI, which was skewed to the right. In this case, a logNFI was used for statistical purposes, because this transformation fit a normal distribution. The variable EDSS score is an ordinal variable. For statistical comparisons of the variables studied among different groups, generalized estimating equation (GEE) regression models were used to account for within-patient and inter-eye correlations (16). The GEE regression model allows adjustment for patient age and gender. The partial Pearson product-moment correlation test and the partial Spearman Rank correlation tests were used to study the correlation between variables RNFL, NFI, TSNIT, BCR, and EDSS. Because no statistical differences were found between values from both eyes of each subject, the average was used instead. The correlation between pairs was obtained after controlling for age and gender. Correla-tion was considered to be significant for P , 0.05. RESULTS RNFL of MS Eyes vs Control Eyes We compared the RNFL measured with OCT and GDx between eyes of patients with MS and controls. Table 1 shows the average RNFL thickness, the logNFI, and the TSNIT average of both groups. There were significant dif-ferences (GEE, P , 0.001) between both groups for all 3 measurements. MS eyes had a significantly thinner RNFL than control eyes. RNFL and MS Subtypes To assess the relationship between RNFL and each MS subtype, we looked for statistical differences of RNFL thickness obtained with both OCT and GDx among the 3 MS subtypes (CIS, RR-MS, and SP-MS). There was a significant progressive reduction in OCT average RNFL thickness from CIS to SP-MS types (GEE, P = 0.008) (See Supplemental Digital Content, Table E1, http://links.lww.com/wno/a86). Differen-ces were not found for logNFI or TSNIT. We classified the RR-MS subtype into benign and nonbenign. There were significant differences (GEE, P , 0.05) between both groups (See Supplemental Digital Con-tent, Table E2, http://links.lww.com/wno/a86). All measure-ments showed that patients with nonbenign RR-MS had significantly thinner layers than patients with benign RR-MS. RNFL of Eyes Without ON and Eyes With ON We compared RNFL thickness between eyes with ON (n = 123) and eyes without ON (n = 53). Eyes with a history of ON showed significantly thinner RNFL than eyes with no history of ON (average RNFL, logNFI, and TSNIT) (See Supplemental Digital Content, Table E3, http://links.lww.com/wno/a86). BCR and MS To assess the effect of MS on brain atrophy, we compared the BCR of patients with MS and controls. Additionally, we compared the BCR in patients with RR-MS judged to be benign vs nonbenign MS. Mean BCR was significantly higher reflecting more brain atrophy in patients with MS (0.12 ± 0.03) than in controls (0.08 ± 0.009) (GEE, P , 0.001) (Table 2). There was no statistical differences between BCR in patients with nonbenign RR-MS and patients with benign RR-MS (GEE, P = 0.103). EDSS and MS We compared the EDSS score among the 3 MS subtypes, and between benign and nonbenign RR-MS. All compar-isons showed significant differences (GEE, P , 0.001) (See Supplemental Digital Content, Table E4, http://links. lww.com/wno/a86). MS subtypes showed a progressive impairment of the EDSS score from CIS (median 1.0) to TABLE 1. Comparisons of OCT average RNFL thickness, logNFI, and TSNIT average between MS eyes and control eyes MS Eyes Control Eyes GEE P OCT: average RNFL thickness, mm 84.51 (14.27) 98.44 (6.83) ,0.001 GDx: logNFI 1.39 (0.22) 1.23 (0.25) ,0.001 GDx: TSNIT average, mm 49.10 (7.76) 53.96 (5.81) ,0.001 Number of eyes 176 118 - Values are mean and SD. GEE, generalized estimating equation; MS, multiple sclerosis; NFI, nerve fiber index; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; TSNIT, temporal-superior-nasal-inferior-temporal average RNFL thickness. Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 25 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. SP-MS (median 4.5). Patients with nonbenign MS had a higher EDSS score (median 4.5) than patients with benign MS (median 1.5) (GEE, P , 0.001). No statistical differ-ences of EDSS score were found between patients with MS without ON and those with ON. Relationship Among OCT, GDx, EDSS, BCR, and Duration of Disease To detect any possible relationship among the parameters measured, correlations among OCT RNFL thickness, logNFI, TSNIT, EDSS, BCR, and disease duration were calculated using the partial correlation test (Pearson product moment or the Spearman Rank). Only patients without history of ON were included in this analysis because this condition may alter RNFL thickness. We used the average between both eyes for each patient. Because each patient had RNFL measurements for the left and right eyes, we compared the group of eyes with thinner RNFL with the contralateral group of eyes. There was no significant difference (P = 0.11) between the OCT RNFL thickness in the group of eyes with thicker RNFL (92.36 ± 10.6 mm) and the group of eyes with thinner RNFL (88.76 ± 10.87 mm). A total of 40 patients were included in this analysis. Significant correlations were found between OCT average RNFL thickness and BCR, EDSS score, and disease duration (Table 3). GDx logNFI was significantly corre-lated with BCR. TSNIT correlated with BCR. EDSS score showed a significant correlation with BCR. BCR correlated with EDSS score and disease duration. Specific data are given in Supplemental Digital Content, Figure E1, http://links.lww.com/wno/a87. DISCUSSION In this study, we investigated the use of OCT and GDx to assess several parameters related to the RNFL thickness in patients with MS and looked for a correlation of those parameters with cerebral atrophy and disability score. We found that the brain atrophy index BCR was higher in patients with MS than in controls and that it correlated with average RNFL thickness, logNFI, and TSNIT. A significant correlation also was found between the average RNFL thickness and EDSS. In our study, eyes of patients with MS had significantly thinner RNFL than eyes of control subjects. The amount of reduction in the RNFL thickness appeared related to the subtype of MS (See Supplemental Digital Content, Table E1, http://links.lww.com/wno/a86). Additionally, within the RR-MS patient group, we found that patients with nonbenign MS had thinner RNFL than benign patients with MS (See Supplemental Digital Content, Table E2, http://links.lww.com/wno/a86). These findings suggest that the severity of the RR-MS may be because of an increased loss of axons within the central nervous system. Substantial reduction in RNFL thickness after a clinical episode of acute has been reported previously (17). In agree-ment with these reports, all measurements in our study showed significantly thinner RNFL in eyes with history of ON than eyes with no history of ON (see Supplemental Digital Content, Table E3, http://links.lww.com/wno/a86). As shown in Table 2, our results for brain atrophy, as assessed by the BCR, are consistent with other studies using various MRI methods for evaluation of brain atrophy (18). TABLE 2. Comparisons of BCR between patients with MS and control subjects, and patients with benign and nonbenign RR-MS Patients With MS Controls GEE P Patients With Nonbenign RR-MS Patients With Benign RR-MS GEE P BCR 0.12 (0.03) 0.08 (0.009) ,0.001 0.14 (0.37) 0.12 (0.02) 0.103 Patients 83 59 - 14 17 Values are mean and SD. BCR, bicaudate ratio; GEE, generalized estimating equation; MS, multiple sclerosis; RR-MS, relapsing-remitting multiple sclerosis. TABLE 3. Correlations between OCT average RNFL thickness, logNFI, TSNIT average, EDSS, and BCR and disease duration in patients with MS without optic neuritis BCR EDSS Score Disease Duration OCT average RNFL thickness, mm 20.48 (P = 0.002) 20.43 (P = 0.003) 20.48 (P = 0.002) GDx: logNFI 0.39 (P = 0.01) - - GDx: TSNIT average, mm 20.34 (P = 0.03) - - EDSS score 0.33 (P = 0.04) - - BCR - 0.33 (P = 0.04) 0.40 (P = 0.01) Values are correlation coefficient and P value (within brackets). Dashes indicate that there was no statistical significant correlation. BCR, bicaudate ratio; EDSS, expanded disability scale score; MS, multiple sclerosis; NFI, nerve fiber index; OCT, optical coherence tomography; RNFL, retinal nerve fiber layer; TSNIT, temporal-superior-nasal-inferior-temporal average; RNFL index. 26 Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. BCR was higher (more atrophy) in patients with MS (0.12 ± 0.03) than in control subjects (0.08 ± 0.009). Ber-mel et al (10) found similar results in MS (0.11 ± 0.02) compared to control subjects (0.09 ± 0.02). BCR also correlated with average RNFL thickness (r = 20.48, P = 0.002; See Table 3 and Supplemental Dig-ital Content, Figure E1A, http://links.lww.com/wno/a87) in patients with MS without ON, whereas this correlation was not present in patients with ON. Siger et al (19) also failed to find any correlation between average thickness RNFL and BCR in patients with MS with ON. This suggests that axonal loss in the retina resulting from ON occurs independently from brain le-sions and atrophy. Similarly, we observed that BCR correlates with TSNIT average (r = 20.34, P = 0.03; See Table 3 and Supplemental Digital Content, Figure E1E, http://links.lww.com/wno/ a87) and also with logNFI (r = 0.39, P = 0.01; See Table 3 and Supplemental Digital Content, Figure E1D, http:// links.lww.com/wno/a87), both being assessed with GDx. There are only a few studies assessing the relationship between GDx and brain MRI measurements. Frohman et al (20) found no correlation between normalized brain volume and TSNIT average RNFL thickness. Several studies have observed a relationship between brain atrophy and EDSS or disease duration. We found that BCR was correlated with EDSS (r = 0.33, P = 0.04; See Table 3 and Supplemental Digital Content, Figure E1F, http://links.lww.com/wno/a87) and with disease duration (r = 0.40, P = 0.01; See Table 3 and Supplemental Digital Content, Figure E1G, http://links.lww.com/wno/a87). This is in agreement with Sanfilipo et al (11) who found that higher EDSS values were associated with higher BCR, but not with the findings reported by Bermel et al (10) who found no significant correlation between EDSS and BCR. Disease duration and neurological disability are 2 clinical parameters linked with progressive axonal loss. In this study, we found that OCT average RNFL thickness showed a significant correlation with EDSS (r = 20.43, P = 0.003; See Table 3 and Supplemental Digital Con-tent, Figure E1B, http://links.lww.com/wno/a87) and with disease duration (r = 20.48, P = 0.002; See Table 3 and Supplemental Digital Content, Figure E1C, http://links.lww.com/wno/a87). Patients with longer disease duration and higher disability had thinner RNFL. Correlation between RNFL thickness and EDSS has been reported in some studies (21,22), but not in others (23). We did not find a significant correlation between the GDx parameters (TSNIT average, logNFI) and EDSS or disease duration, as reported by Siepman et al (21). OCT and GDx provide information on different aspects of RNFL structure. OCT provides thickness values similar to histology whereas GDx assesses birefringence of microtubules. Frohman et al (20) observed that MRI measurements and EDSS had a much stronger relationship with OCT RNFL than with GDx, and our results are in agreement with this observation. This suggests that OCT might better reflect the neurodegenerative process of MS compared to GDx. REFERENCES 1. Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634-642. 2. Waxman SG, Black JA. Retinal involvement in multiple sclerosis. Neurology. 2007;69:1562-1563. 3. Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, Plant GT, Miller DH. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383-391. 4. Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113: 324-332. 5. Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488-1494. 6. Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway- Heath DF, Plant GT, Miller DH. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain. 2008;131:277-287. 7. Zivadinov R, Leist TP. Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging. 2005;15:10-21. 8. Aylward EH, Schwartz J, Machlin S, Pearlson G. Bicaudate ratio as a measure of caudate volume on MR images. AJNR Am J Neuroradiol. 1991;12:1217-1222. 9. Doraiswamy PM, Patterson L, Na C, Husain MM, Boyko O, McDonald WM, Krishnan KR. Bicaudate index on magnetic resonance imaging: effects of normal aging. J Geriatr Psychiatry Neurol. 1994;7:13-17. 10. Bermel RA, Bakshi R, Tjoa C, Puli SR, Jacobs L, Bicaudate ratio as a magnetic resonance imaging marker of brain atrophy in multiple sclerosis. Arch Neurol. 2002;59:275-280. 11. Sanfilipo MP, Benedict RH, Sharma J, Weinstock-Guttman B, Bakshi R. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage. 2005;26:1068-1077. 12. Butzkueven H, Kolbe SC, Jolley DJ, Brown JY, Cook MJ, van der Mei IA, Groom PS, Carey J, Eckholdt J, Rubio JP, Taylor BV, Mitchell PJ, Egan GF, Kilpatrick TJ. Validation of linear cerebral atrophy markers in multiple sclerosis. J Clin Neurosci. 2008;15:130-137. 13. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452. 14. Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13: 227-231. 15. Ramsaransing G, Maurits N, Zwanikken C, De Keyser J. Early prediction of a benign course of multiple sclerosis on clinical grounds: a systematic review. Mult Scler. 2001;7:345-347. 16. Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford University Press, 2002. 17. Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963-969. 18. Sharma J, Sanfilipo MP, Benedict RH, Weinstock-Guttman B, Munschauer FE III, Bakshi R. Whole-brain atrophy in multiple sclerosis measured by automated versus semiautomated MR imaging segmentation. AJNR Am J Neuroradiol. 2004;25: 985-996. Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 27 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. 19. Siger M, Dziegielewski K, Jasek L, Bieniek M, Nicpan A, Nawrocki J, Selmaj K. Optical coherence tomography in multiple sclerosis: thickness of the retinal nerve fiber layer as a potential measure of axonal loss and brain atrophy. J Neurol. 2008;255:1555-1560. 20. Frohman EM, Dwyer MG, Frohman T, Cox JL, Salter A, Greenberg BM, Hussein S, Conger A, Calabresi P, Balcer LJ, Zivadinov R. Relationship of optic nerve and brain conventional and non-conventional MRI measures and retinal nerve fiber layer thickness, as assessed by OCT and GDx: a pilot study. J Neurol Sci. 2009;282:96-105. 21. Siepman TA, Bettink-Remeijer MW, Hintzen RQ. Retinal nerve fiber layer thickness in subgroups of multiple sclerosis, measured by optical coherence tomography and scanning laser polarimetry. J Neurol. 2010;257:1654-1660. 22. Toledo J, Sepulcre J, Salinas-Alaman A, García-Layana A, Murie- Fernandez M, Bejarano B, Villoslada P. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906-912. 23. Oreja-Guevara C, Noval S, Manzano B, Diez-Tejedor E. Optic neuritis, multiple sclerosis-related or not: structural and functional study [in Spanish]. Neurologia. 2010;25:78-82. 28 Abalo-Lojo et al: J Neuro-Ophthalmol 2014; 34: 23-28 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |