| OCR Text |
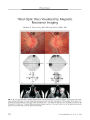
Show STATE OF THE ART Advances in Imaging of the Optic Disc and Retinal Nerve Fiber Layer Gary L. Trick, PhD, Fadi Y. Calotti, MD, and Barry Skarf, PhD, MD Abstract: In the past decade, three technologies for imaging the optic disc and retinal nerve fiber layer have become commercially available: 1) confocal scanning laser tomography with the Heidelberg retinal tomograph; 2) confocal scanning laser polarimetry with the GDx VCC; and 3) optical coherence tomography with the Stratus OCT. Each uses different principles of physics. Understanding the merits and limitations of each of these technologies requires familiarity with the principles of operation of each device. This knowledge should be considered a prerequisite for the appropriate clinical utilization of these devices and for accurate interpretation of their results. (/ Neuro- Ophthalmol 2006; 26: 284- 295) Three morphologic characteristics of the optic disc are fundamental indicators of optic nerve health and disease: 1) excavation ( or cupping); 2) elevation or swelling; and 3) pallor. Equally important in assessing the health of the optic nerve is the evaluation of the peripapillary retinal nerve fiber layer ( RNFL) for features such as thickness, focal defects ( gaps, streaks, or thinning), and hemorrhages. These characteristics must be evaluated and graded during the diagnosis of glaucomatous and non- glaucomatous optic neuropathies and when monitoring the progression and evolution of optic nerve disease. Until several years ago, these evaluations were performed clinically and qualitatively by ophthalmoscopy or by examining and comparing stereoscopic fundus photographs and red- free images ( for RNFL). In the past decade, new technologies for imaging the optic disc and retina that provide objective quantitative estimates of these morphologic features have been developed. Ophthalmologists, particularly those specializing in neuro- ophthalmology and glaucoma, now have access Departments of Ophthalmology, Henry Ford Hospital, Detroit, Michigan ( GLT, FYC, BS); and Anatomy and Cell Biology, Wayne State University, Detroit, Michigan ( GLT). Address correspondence to Gary L. Trick, PhD, Department of Eye Care Services, Henry Ford Health Science Center, 2799 West Grand Blvd., Detroit, MI 48202; E- mail: GLTrickl@ aol. com to instruments that generate data concerning various properties of the optic disc and peripapillary retina ( Table 1 and Fig. 1). Three technologies for imaging the optic disc and retina are available commercially ( Fig. 1): 1) confocal scanning laser tomography ( CSLT) with the Heidelberg retinal tomograph ( Heidelberg Engineering GmbH, Dos-senheim, Germany); 2) confocal scanning laser polarimetry ( CSLP) with the GDx VCC ( developed by Laser Diagnostic Technologies, now part of Carl Zeiss Meditec Inc., Dublin, CA); and 3) optical coherence tomography ( OCT) with the Stratus OCT ( Carl Zeiss Meditec Inc.). Each uses different principles of physics to achieve the goal of producing objective, quantitative and reproducible images of the optic disc, RNFL, and macula along with an analysis of morphologic features. Understanding the merits and limitations of each of these technologies requires familiarity with the principles of operation of each device. This knowledge should be considered a prerequisite for the appropriate clinical utilization of these devices and for accurate interpretation of their results. But the essential requirement for the neuro-ophthalmologist is that the information acquired truly represent the condition of the optic nerve and RNFL and that the findings be reliable, reproducible, and useful for monitoring changes in disc structure and RNFL thickness. Because RNFL and optic disc evaluation play such a critical role in the care of patients with glaucoma, development of these technologies was largely motivated by the need for a more objective measure of optic disc structure in these patients and in patients with suspected glaucoma. However, neuro- ophthalmologists are interested in a set of different, though overlapping, parameters. Elevation and edema ( swelling of the optic disc), and the stability, evolution, or progression of these features are frequent concerns. Optic disc and neuroretinal rim color is a critical feature in the evaluation of non- glaucomatous optic atrophy, and neuroretinal rim pallor is a distinguishing feature that helps to differentiate non- glaucomatous atrophy from glaucoma. The peripapillary nerve fiber layer may swell or thicken when the optic disc is edematous or when the retina is ischemic and peripapillary nerve fiber layer height 284 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 TABLE 1. Characteristics of fundus imaging technologies Instrument Manufacturer Current Version Technologic Principle Illumination Source Imaging Technique RNFL Thickness Measurement Area Scan Depth Focus Range Pixelization Optical Resolution Digital Resolution Image Acquisition Time HRT Heidelberg Engineering, Heidelberg, Germany HRT III Confocal Scanning Laser Ophthalmoscope 670 nm diode laser Constructs a 3- dimensional image of the optic disc and surrounding peripapillary RNFL surface Estimated from Height above reference plane 15° X 15° 1.0 to 4.0 mm - 12 to + 12 diopters 384 X 384 Transverse: 10 | xm Longitudinal: 300 | xm 30 | xm 0.4 to 1.5 seconds RNFL, retinal nerve fiber layer. NA = Not available. GDx Carl Zeiss Meditec Inc, Dublin, California GDx VCC Scanning Laser Polarimeter ( Birefringence) GaAIAs laser diode, 780 nm nominal value Uses reflected laser light to image the retina and polarimeter to measure birefringence of the RNFL at each point imaged Estimated from the phase shift in the incident polarized light cause by microtubule birefringence 20° X 20° 2.0 mm - 10 to + 5 diopters 128 X 128 NA 5 | xm Less than one second OCT Carl Zeiss Meditec Inc, Dublin, California Stratus 3 Interferometry Superluminescent Diode, 820 nm Estimated from algorithm that calculates distance of various retinal surfaces revealed by intensity changes due to refractive index changes between layers Measured by an expert algorithm that estimates the boundaries of the highly- reflective layer deep to the retina surface 29° X 23° 2.0 mm in tissue - 20 to + 24 diopters Axial: 1024 X Transverse: 128- < 10 | xm in tissue NA 2 seconds - 768 ( elevation) may change without any real quantitative change in the retinal ganglion cell or axonal components of the retina itself. Under these conditions changes in RNFL thickness may not represent actual changes in the viability of the retinal nerve fibers. Despite differences in technology as well as differences in the optic disc and retinal characteristics that are measured, the new imaging technologies are now being used in the evaluation of non- glaucomatous optic nerve disorders, including non- glaucomatous optic neuropathies that cause disc excavation, swelling, or pallor, as well as retinal disorders, including macular edema, macular holes, and pseudoholes, epiretinal membranes, and central serous chorioretinopathy. However, the usefulness of the images and the concomitant data obtained in patients with non-glaucomatous optic nerve disease is still largely unclear, since published studies examining the efficacy of the new imaging technologies in these conditions are quite limited. CONFOCAL SCANNING LASER TOMOGRAPHY CSLT is based on the technology used in the scanning laser ophthalmoscope: a small diameter laser beam rapidly scans the retinal surface horizontally and vertically ( Fig. 2). With CSLT, the light reflected back from each illuminated point passes through a small aperture ( pinhole diaphragm) that is positioned so that the focal plane of the retina and the focal plane of the light sensor are optically conjugate. This optical design ensures that only light from the retinal plane being scanned is focused on the detector, whereas scattered 285 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Trick et al FIG. 1. Commercially available computerized devices for imaging the optic disc and retina. A. Heidelberg retinal tomograph ( HRT II). ( Courtesy of Heidelberg Engineering GmbH.) B. GDx access nerve fiber layer analyzer. ( Courtesy of Carl Zeiss Meditec.) C. Stratus OCT 3. ( Courtesy of Carl Zeiss Meditec.) light and reflected light originating from planes that are out of focus do not enter the detector. A series of confocal images are then collected at sequential axial depths, aligned appropriately, and processed to calculate a 3 - dimensional representation of the surface topography of the retina or optic disc. Although several confocal scanning systems have been available commercially, the Heidelberg retinal tomograph ( HRT) has become the most widely used system. The original HRT ( HRT I) and the newer, more compact HRT II ( Fig. 1A) use a 670 nm diode laser that rapidly scans the retina point by point in the x- and j- axes. A third version, the HRT III, is now available, but because there is limited information on the clinical utility of this instrument, it will not be discussed in this review. Each HRT image is a composite of measurements from an array of 256 by 256 pixels ( HRT I) or 384 by 384 pixels ( HRT II). The devices collect a series of confocal 2- dimensional images at sequential axial depths ( z- axis) extending from the anterior surface of the optic disc to the retrolaminar portion of the disc. Total scan depth can range from 1 to 4 mm. The HRT I scans images at 32 levels, and the HRT II can be programmed to collect image data from 16 to 64 levels. The HRT software then aligns the series of confocal images to produce a three- dimensional representation of the surface topography. Image accuracy is improved when three data sets ( each representing one complete three- dimensional image) are averaged to produce a mean topographic image ( 1), and it has now become fairly standard for users to collect and average three successive images of the optic disc. The field of view is fixed at 15 by 15° and the spatial resolution is 10 \ x, m per pixel. The HRT provides 3- dimensional topographic images of the optic disc and adjacent nerve fiber layer. The software automatically calculates a series of " stereometric" values, including disc area, cup area, cup depth, cup volume, rim area, rim volume, linear cup- to- disc ratio, and cup- to-disc area ratio. These calculations are based on an arbitrary reference plane 50 | Jim below the surface of the papillomac-ular bundle and on an operator- delineated contour line that outlines the optic disc margin. As the reference plane intersects the surface contour of the disc, all structures between the reference plane and the underlying disc surface are considered to belong to the optic cup ( red) and all structures above the reference plane and within the contour line defining the disc margin are considered to belong to neuroretinal rim ( blue). Measures independent of the reference plane are mean and maximum cup depth. The software also makes use of the stereometric measures to calculate a " cup shape measure" and to classify the optic disc topography as either " normal" or " glaucomatous." Significant changes in the stereometric measures, obtained serially over time, are used to monitor progression of disease. Results are highly reproducible and are considered accurate for following topographic changes ( 2- 4). To estimate RNFL thickness the HRT software measures the retinal height along a contour surrounding the disc and subtracts the height of the reference plane. This is shown graphically as a graph of contour line height against angular position around the disc circumference. Thus, the HRT measures surface topography ( elevation) directly and only estimates RNFL thickness indirectly. The software also calculates a mean RNFL thickness and RNFL cross- sectional area. SCANNING LASER POLARIMETRY Scanning laser polarimetry ( SLP) is designed to provide only a measure of nerve fiber layer thickness. Like CSLT, SLP uses a confocal scanning laser ophthalmoscope 286 © 2006 Lippincott Williams & Wilkins Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Detector Pinhole aperture Retina FIG. 2. Confocal scanning laser ophthalmoscope. Light from the laser source enters the eye and is reflected back from the retina. Optics are arranged so that only light from the focal plane in the retina can be imaged through the pinhole aperture to be detected by the light detector. Light originating from other planes that are out of focus ( broken lines) is blocked by the pinhole aperture. to image the optic disc and peripapillary nerve fiber layer, but it also incorporates a retinal polarimeter that measures changes in the polarization of light reflected back to the instrument from the retina ( Fig. 3). The most current commercial version of the scanning laser polarimeter is the GDx VCC ( Fig. IB), which uses a 785 nm diode laser. The natural birefringence of the linearly arrayed, parallel microtubules in the RNFL produces a change in the polarization properties of the laser light source when it passes through the RNFL. This change or phase shift in polarization is called " retardation." The polarization state of the light reflected from the eye is measured by the polarimeter and is displayed as a 256 by 128 pixel retardation map. Because the retardation is linearly correlated with the thickness of the polarizing medium ( the RNFL microtubules), the retardation at each image point can be used as a direct index of the RNFL thickness at each point in the image. A problem with this technology is that any bire-fringent structure through which the laser beam passes will alter the retardation of the beam. In the eye, the cornea and crystalline lens have significant birefringence that is highly variable among individuals ( 5,6). Early versions of the scanning laser polarimeter included a " fixed corneal compensator" to correct for the expected natural birefringence of the cornea and lens. However, that fixed compensation was not appropriate for all individuals and produced results that were sometimes difficult to interpret. The current version of the GDx estimates the anterior segment birefringence individually for each subject tested. The measures from the peripapillary retina are then corrected for the anterior segment birefringence determined for that individual. This routine, called a " variable corneal compensator" ( VCC) by the manufacturer, makes use of the fact that the averaged birefringence of the macula is very low, so that the overall retardation measured when imaging the macula is derived almost entirely from anterior segment structures. Thus, the GDx VCC estimates and corrects for the retardation due to anterior segment birefringence by determining the overall retardation from imaging the macula and correcting the retardation from the peripapillary retina by that amount. Several studies have reported that correction for corneal birefringence with the VCC increases the discriminating power of this technology in detection of early to moderate glaucoma ( 7,8). With the GDx, the software automatically fits an ellipse to the optic disc margin. However, the operator is required to confirm the size and position of the ellipse. This outline is used by the software to generate a 3.2 mm diameter, 8- pixel wide circle centered on the optic disc and used for RNFL analysis. The color printout of the GDx includes an image of the area scanned by the laser alongside a color- coded nerve fiber layer thickness map based on the retardation measured at each point. Red, yellow, and orange 287 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Trick et al Crossed Detector Rotating Half- Wave Plate FIG. 3. Scanning laser polarimeter ( SLP). A 785 nm laser diode light passes through a linear polarizer and is rotated by the half- wave plate into 20 successive input polarizations. The beam is focused onto a point on the retina and reflected back from the retina, passing through the same optical structures a second time until reaching the polarizing beam splitter. The corneal and retinal birefringence are in series and both cause a retardation of the polarized light as it traverses tissues. If the retardation introduced by the cornea is well compensated, the measured retardation can be attributed to the RNFL and is considered to be proportional to its thickness. At the polarizing beam splitter, the light returning from the eye is separated into two channels. The light in the parallel channel passes through the detector for parallel polarization; the light in the perpendicular channel passes through the detector for crossed ( perpendicular) polarization. The amount of light entering the two detectors is measured for each of the 20 different polarization steps generated by the half- wave plate as it rotates through 90°. This results in a series of 40 images with different input polarization states ( 20 from each of the two channels). These 40 images are used to generate the final output images that vary according to their polarization content. Retina represent regions of higher retardation, whereas blue and black indicate areas of lower retardation. The GDx printout also includes a graph that plots RNFL cross- sectional thickness around the 360° circle from temporal to superior to nasal to inferior and back to temporal ( the TSNIT graph). In normal individuals, this thickness graph has a typical " double- hump" contour, indicating the variation in RNFL thickness around the disc. The shaded area of this graph includes 95% of normal values. A deviation map similar to the deviation plot on the Humphrey visual field analyzer is also provided. The deviation map compares the RNFL thickness at each of the points included in the map of the 20° by 20° area centered on the optic disc to the normative database and indicates which areas fall below the normal limit. These defects are displayed in a color scheme based on probability. The GDx software generates five summary parameters ( the number, ellipse average, superior average, inferior average, and ellipse standard deviation) that are compared to a normative database. The software indicates whether the parameter value falls within the normal range for the patient's age. One parameter, the number, is based on a neural network approach that evaluates the entire 20° by 20° thickness map. This parameter is intended to reflect the overall status of the RNFL and provides a value between 0 and 100. Values < 30 are considered normal, whereas values between 31 and 50 are suspicious for nerve fiber loss and values > 50 indicate definite nerve fiber layer thinning. The computer algorithm also calculates average quadrantic measurements, measurement ratios, symmetry measurements between superior and inferior quadrants, and modulation parameters. The software can also print out a serial 288 © 2006 Lippincott Williams & Wilkins Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 analysis of successive, sequential tests performed on the same patient over time. OPTICAL COHERENCE TOMOGRAPHY OCT generates high resolution, 2- dimensional, cross-sectional images of the internal microstructure of posterior ocular structures, including the RNFL, optic disc, and macula. Transverse images of the retina are produced using " low coherence tomography," an optical measuring technique that is analogous to B- scan ultrasound except that, in place of sound waves, OCT uses a laser- generated beam of light. The commercially available Stratus OCT 3 ( Fig. 1C) is a non- contact, transpupillary imaging device that can produce images with an axial resolution of approximately 10 | xm. A near- infrared ( 820 nm) light beam, generated by a superluminescent diode laser, is directed into the eye through a conventional slit- lamp biomicroscope and focused on the retinal tissue using a 78- diopter condensing lens ( Fig 4). The retinal microstructure is assessed by measuring the temporal delay of the back- scattered infrared light. The delay is measured by a fiberoptic Michelson interferometer that operates by creating interference between the back- scattered light from the tissue and a beam of light from a variable length reference arm. An infrared camera is used to view the fundus and guide image acquisition. The Stratus OCT 3 makes 128 to 768 axial samples ( so- called " A- scans") in a single scan. A series of successive A- scans are then combined into one image ( a so- called " B- scan") with a resolution of approximately 10 | xm vertically and 20 | xm horizontally. The OCT image obtained from this process is presented in pseudo- color with the visible spectrum corresponding to the optical reflectivity of tissue interfaces. Darker colors such as blue or black represent regions of little optical reflectivity, whereas brighter colors such as red and white represent greater reflectivity. Generally, nerve fiber and plexiform layers, which consist of axonal structures, are highly reflective and appear red in the OCT images. Nuclear layers are weakly reflective and appear blue- black. In imaging of the retina, a highly reflective layer is identified posteriorly, corresponding to the retinal pigment epithelium ( RPE) and choriocapillaris. This layer typically measures approximately 70 | xm and serves as the posterior boundary for the measurement of neurosensory retinal thickness. Just anterior to the RPE/ choriocapillaris band, a darker ( less reflective) band corresponds to photoreceptor outer segments. Middle retinal layers exhibit moderate reflectivity. The vitreoretinal interface is defined by the contrast of the non- reflective vitreous against the more reflective retinal structures. Normal central macular thickness measures 147 ± 17 | xm. The fovea is identified DETECTION ELECTRONICS FIBEROPTIC BEAMSPLITTER SLIT LAMP h COMPUTER jjjyu^ REFLECTED BEAM FIG. 4. Stratus optical coherence tomography. The light source is a superluminescent diode that is coupled to a fiberoptic nterferometer ( beam splitter) that creates two paths for the beam of light. One path is for the reference beam, which is reflected off a mirror that is positioned at a variable, known distance, producing a known time delay in the reflected beam of light. The second path is directed into the eye via a probe module that scans the beam transversely across a portion of ocular fundus. The backscattered ( reflected) light from the fundus consists of multiple reflections with different time delays and intensities, depending on the structure of the tissue scanned. This light signal is then routed back to the interferometer where it is combined with the light reflected back from the reference mirror. The strength of the signal from the interferometer is measured by a photosensitive detector. The various components in the backscattered light signal, each corresponding to tissue structure at a particular depth, are measured by varying the position of the reference mirror, thus producing different time delays for the reference light, while processing the detector output. The output then represents the intensity of the reflected light versus distance along the z- axis, analogous to A- mode ultrasound scanning. 289 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Trick et al by its characteristic central depression. Blood vessels both within the retina and the choroid are highly light- scattering and they attenuate the signal for deeper structures, producing shadowing effects. Signal attenuation by the retina and RPE/ choriocapillaris weakens reflections from the deeper choroid and sclera, limiting the OCT imaging depth. If the optic disc is included in the scan, its outer margin can be detected by the termination of the highly reflective RPE/ choriocapillaris layer at the lamina cribrosa. The vitreoretinal interface over the disc, which is the boundary between the poorly reflective vitreous and the highly reflective nerve fiber layer, defines the surface of the disc and outlines its contour. As the retinal nerve fibers bend toward the lamina cribrosa and become less perpendicular to the incident optical beam, their reflectivity decreases. Because the OCT can be used to obtain cross-sectional images of the optic disc at any orientation, multiple radial scans through the center of the optic disc can be used to create a cross- sectional profile of the entire disc. Optic disc diameter, cup size ( volume, area, or diameter), and area or width of the neuroretinal rim can be calculated from these data using expert algorithms. These algorithms determine the disc margin as the series of points at which the photoreceptor layer, RPE, and choriocapillaris terminate. This contour must be inspected and confirmed by a human observer/ operator. The disc diameter at a given axis is then the distance between margins on opposite sides of the disc. The edge or boundary of the cup is estimated by constructing a line parallel, but anterior, to the line denning the disc diameter. This line is offset anteriorly by a standard amount and the points where it intersects the surface of the disc are denned as the edge of the cup. Similar lines at multiple axes define the entire contour of the cup. Circular tomograms of the peripapillary retina are used to document RNFL thickness at a fixed distance around the disc. Typically, a circle centered on the optic disc with a diameter of 3.4 mm is used. The circumpapillary OCT is displayed " unwrapped" as a linear TSNIT image. The RNFL in these images appears as a highly reflective red layer just inside the vitreoretinal interface, which is assumed to be its anterior boundary. The posterior boundary of the RNFL is determined to be the depth at which the signal intensity of the optical backscattering crosses a set threshold level. Computer algorithms can determine these points and plot nerve fiber layer thickness from these images. The OCT data are usually displayed as a graph of RNFL thickness against position around the optic disc. The analysis also produces average values of RNFL thickness in each of the four quadrants around the disc and for each of the twelve 30° sectors. An average RNFL thickness around the entire disc is also calculated. A normative, age- adjusted, database is provided for comparison. As expected, the thickness of the highly reflective RNFL varies around the optic disc. COMPARING THE INSTRUMENTS CSLT, SLP, and OCT share a number of common features. They each provide objective, quantitative measures of optic disc structure and RNFL thickness. The instrument for each measures parameters and calculates indices that can be used to monitor morphologic change ( and, presumably, disease progression) objectively over time. However, each technology has distinct merits and limitations. Operator Interaction The accuracy of the optic disc and RNFL measurements made with any of these devices is dependent on the care with which the images are acquired. Each instrument provides the operator with " quality control" feedback. Even so, the operator must have a basic understanding of optic disc and RNFL anatomy as well as knowledge of optical imaging. For example, the HRT and the GDx require the operator to place or evaluate a contour line around the optic disc. The accuracy of HRT and GDx measurements will be highly dependent on the precision with which the contour line is drawn. Operator input introduces a subjective component to the HRT and GDx analysis that can be a source of error. In the HRT, the contour line is used to define the reference plane. Consequently, all measures based on the reference plane will be influenced by the accuracy of the contour line. In the GDx, contour line placement is somewhat less critical because the deviation map is independent of the ellipse. Nevertheless, in both cases the contour line should be placed with care because it is drawn only on the initial ( baseline) image. The software will import the contour line into the subsequent images; imprecisely drawn contour lines on baseline images can result in the propagation of error. Measurement of Optic Disc and RNFL Parameters CSLT is an excellent technique for measuring optic disc topography. However, with the HRT, RNFL parameters are estimated on the basis of placement of the contour line and the assumed reference plane. Because SLP and OCT measure tissue thickness more directly, the RNFL thickness measured by these methods is not dependent upon an appropriate choice of a reference plane. Limitations shared by all three technologies are the inability to detect shifts in the positions of the retinal vessels, changes in optic disc pallor, or evolution of disc hemorrhages, features often associated with disease progression. 290 © 2006 Lippincott Williams & Wilkins Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Sensitivity and Specificity Although many studies have measured the sensitivity and specificity of each of these devices, primarily in patients with glaucoma, relatively few studies have compared the sensitivity and specificity of all three devices to each other. Sanchez- Galeana et al ( 8) compared the HRT, GDx, and OCT in 50 normal subjects and 39 patients with glaucoma with early to moderate visual field damage. For the HRT, they found a sensitivity from 64% to 75% ( depending on the degree of glaucoma) and a specificity of 68% to 80%. For the GDx, sensitivity ranged from 72% to 82% and specificity from 56% to 82%. For the OCT, sensitivity ranged from 76% to 79% and specificity from 68% to 81%. Greaney et al ( 9) compared the HRT, GDx, and OCT in 89 normal and glaucomatous eyes. They found comparable sensitivities and specificities for all three instruments: HRT ( 0.94% and 0.90%), GDx ( 0.89% and 0.87%), and OCT ( 0.82% and 0.84%). Thus, it appears that all three devices have similar sensitivity and specificity for discriminating normal subjects from patients with glaucoma. However, higher sensitivities and specificities are published for each of these devices as there continues to be an evolution of new measurement parameters, the application of new combinations of existing parameters, and re- engineering. As a result, interpretation of the literature must be done cautiously because diverse study samples ( patients with suspected glaucoma or patients with early, moderate, or more advanced glaucoma) are frequently used. In addition, some studies report sensitivity and specificity for a single " optimal" parameter, whereas other studies use various combinations of parameters. There has been no systematic study of the sensitivity and specificity of these devices in non- glaucomatous optic neuropathies. Pupil Dilation Neither the HRT, GDx, nor OCT specifically require pupil dilation for image acquisition. However, Zangwill et al ( 10) showed that HRT image reproducibility improves with dilation, particularly in eyes with small pupils and cataract. Therefore, dilation is recommended whenever pupil size is < 2- 3 mm. GDx images may be obtained through an undilated pupil with a minimum diameter of 2 mm. However, uniformity in pupil size is essential to detect changes in follow- up RNFL measurements. Although the OCT does not require pupil dilation for either macular or optic disc imaging, better reproducibility of most optic disc measurements is obtained with a dilated pupil ( 11). Change Detection HRT strategies for change detection include serial analyses of global and regional topographic indices ( cup- to- disc ratio, cup volume, and cup shape), in which color- coded ( red/ green) significance indicators represent changes relative to baseline. Chauhan et al ( 12) have described a more sophisticated change analysis algorithm based on a probabilistic approach with variability estimates that use clusters of 4 X 4 pixels to create superpixels. Three follow- up images are compared with a baseline image and a change probability map is generated. This algorithm appears to facilitate differentiation between biologic change and test- retest variability. However, it has yet to be validated in a prospective clinical trial. At present, the simplest way to use the HRT parameters for following optic disc topography is to determine whether the difference between the stereometric values of two images taken at different times exceeds the published variability of 5%- 9% for glaucomatous eyes. Unfortunately, comparable variability indices for other optic neuropathies have not been published. GDx strategies for change detection include evaluation of changes in absolute values of retardation measurements, changes in quadrantic RNFL thickness measurements, and changes in the double- hump RNFL thickness profiled. Although a change analysis algorithm exists, statistical units of probability are absent. Thus, biologic change cannot be differentiated from measurement variability. Prospective studies are necessary to validate change analysis strategies. For the OCT, change analysis software has only recently been introduced; thus, no reports have described longitudinal change in patients with disease progression. As presently configured, this algorithm generates a serial analysis of RNFL thickness measurements between two OCT images but statistical units of change probability are not provided. Thus, true biologic change cannot be differentiated from test- retest variability. CLINICAL APPLICATIONS IN NEURO- OPHTHALMOLOGY These technologies have been applied clinically to quantify optic disc and RNFL structure in numerous reported studies and cases. However, the most widespread use has been in patients with glaucoma, suspected glaucoma, or ocular hypertension. Relatively few investigations have targeted non- glaucomatous optic neuropathies. Furthermore, the accumulation of meaningful longitudinal data on patients has been limited by the rapid pace of upgrades and advances in the instruments. Data collected with newer versions of the equipment and software are often not back-wardly compatible with data collected using earlier models of the equipment. Of the three instruments, the HRT has maintained the longest continuously compatible database across platforms. For this reason, the HRT has been the most thoroughly investigated of the three technologies. Nevertheless, longitudinal studies have concentrated almost exclusively on patients with glaucoma or ocular hypertension 291 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Trick et al and on patients with suspected glaucoma. Some results of these studies, such as the reproducibility of measurements and the specificity and sensitivity of the tests, may be relevant to the evaluation of results obtained for other optic neuropathies. Non- Glaucomatous Optic Neuropathies Although limited in scope, studies on the use of the HRT, GDx, or OCT in non- glaucomatous optic neuropathies have appeared. Several reports have shown that the HRT can be used to quantify the magnitude and monitor the progression or resolution of papilledema in patients with idiopathic intracranial hypertension ( IIH) ( 13). The HRT can be used to detect regional differences in topography between IIH patients with papilledema and patients with pseudopapilledema ( 14), as well as to demonstrate differences in the evolution of optic disc swelling between IIH patients with papilledema and patients with anterior ischemic optic neuropathy ( 15). In addition, OCT has been used to demonstrate subretinal fluid accumulation involving the macula in patients with papilledema ( 16). Optic disc topography measured with the HRT at the atrophic stage of Leber hereditary optic neuropathy is similar in morphology to discs in patients with normal tension glaucoma; however, the disc excavation ( cupping) is significantly deeper in normal tension glaucoma ( 17). Peripapillary RNFL thinning in patients with optic nerve head drusen has been measured using OCT ( 18- 21), the GDx ( 19,22,23), and the HRT ( 14,19,24), but there is only one report describing 4 patients with drusen that have been studied using all three technologies ( 19). SLP using the GDx in patients with central retinal artery occlusion shows a diffuse reduction in the retardation, indicating marked loss of birefringence of the RNFL, consistent with loss of the nerve fiber layer or, at least, of its normal birefringent architecture ( 25). The GDx has also been used to study patients with anterior ischemic optic neuropathy ( AION) in whom areas of RNFL thinning correspond to field defects and the amount of thinning correlates with the visual field loss ( 26,27). The HRT was used to compare optic nerve head morphology between patients who suffered arteritic and non- arteritic AION, using their uninvolved contralateral eyes as controls. HRT parameters showed changes consistent with significant enlargement and excavation of the optic cup following arteritic AION only; no such change was observed in eyes that experienced an episode of non- arteritic AION ( 28). Decreased RNFL thickness has been found in patients with demyelinating optic neuritis with the GDx ( 29,30) and with the OCT ( 31,32). However, in patients with band atrophy of the optic nerve and bitemporal visual field defects from chiasmal compression, RNFL thickness measurements with the GDx have been found to be somewhat equivocal, with a failure to detect axonal loss in the region temporal to the optic disc ( 33), as well as some cases of chronic unilateral disc atrophy ( 34). In contrast, the OCT did demonstrate the characteristic pattern of RNFL loss in eyes from a different sample of patients with band atrophy and bitemporal hemianopia due to chiasmal tumors ( 35). The OCT findings in a patient with a traumatic optic tract lesion and a homonymous hemianopia showed marked thinning of the temporal and nasal RNFL in the eye contralateral to the lesion and a substantial decrease in the RNFL inferiorly in the ipsilateral eye, as expected ( 36). GDx ( 37) and OCT ( 38) imaging of one patient each with traumatic optic neuropathy showed marked thinning of the RNFL. OCT showed that the RNFL was thickened in early ( acute) Leber hereditary optic neuropathy ( LHON), whereas it became markedly thinned in atrophic ( late) cases ( 39). Those patients with LHON who recovered vision late had partially preserved RNFL. Another study of unaffected carriers of LHON showed that all genetic subgroups have thickening of the papillomacular bundle and that male carriers have more diffuse involvement than female carriers ( 40). Three patients with toxic optic neuropathy from ethambutol examined with OCT were found to have considerable loss of RNFL, especially temporally ( 41). A case of vigabatrin- induced optic neuropathy imaged with the HRT and GDx confirmed thinning of the RNFL that accompanied constricted visual fields and a reduced electro-retinogram b- wave ( 42). Macular Disease OCT has frequently been used to study changes in retinal structure associated with a wide variety of retinopathies, especially macular diseases. OCT can reliably diagnose true macular holes, lamellar holes, and pseudo-holes ( 43- 46). OCT also accurately shows the presence and extent of epiretinal membranes, which have highly reflective optical properties, the foci of traction, and the degree of associated macular thickening and edema. OCT can be further used to identify, characterize, and quantify intraretinal and subretinal fluid accumulation ( 47- 49) in diabetic retinopathy and venous occlusive disease and track changes in tissue thickness following therapy. OCT can localize subtle fluid collections and differentiate the layers of involvement in central serous chorioretinopathy ( 50,51). In patients with age- related macular degeneration, OCT has shown promise as an adjunctive diagnostic tool in monitoring the clinical response to photodynamic therapy and pharmacotherapy ( 52- 54). The use of the GDx and HRT in these retinal conditions has been extremely limited. Accurate and objective mapping of retinal thickness by the GDx to improve the early diagnosis of macular edema might be 292 © 2006 Lippincott Williams & Wilkins Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 possible if the locus of the edema relative to the fovea could be located and subtle changes in retinal thickness could be detected and quantified. It is also possible that the GDx could be used to differentiate between macular cysts and macular holes and to detect epiretinal membranes and cystoid macular edema although we are unaware of any published studies documenting this possibility. However, the GDx has lower resolution than OCT. Therefore, its primary benefit in visualizing retinal pathologic conditions remains in offering a color- coded posterior pole view that allows accurate localization of the pathologic changes and monitoring the effect of subsequent treatment. Studies in patients with macular edema suggest that HRT has a relatively low sensitivity ( 67%) but high specificity ( 90%) ( 55,56). It has been suggested that the HRT can differentiate between macular holes and pseudoholes on the basis of the contour and depth of the cavity. Similarly, in age- related macular degeneration with choroidal neovas-cular membrane and in central serous chorioretinopathy, HRT diagnosis is based on surface height. However, the HRT II appears to have better agreement with clinical assessment of diabetic macular edema than does a retinal thickness analyzer ( 57). WHERE MATTERS STAND Recent advances in ocular imaging have resulted in technologies that provide a means to obtain accurate, objective, quantitative, and reproducible structural measures of optic disc topography and RNFL thickness. However, in practice, where are we today? Because there are differences in the principles of operation, as well as the advantages and liabilities of each device, no one instrument is best for all purposes. For optic disc imaging, both the HRT and OCT are useful for monitoring changes in optic disc cup excavation and in the area of neuroretinal rim. However, because the HRT measures surface elevation, it is particularly useful for documenting changes in optic disc swelling. Longitudinal data concerning usefulness and reliability in detecting changes and their significance are only available for the HRT, for it is the only technology that has been available long enough to conduct such studies. In neuro- ophthalmology, imaging the RNFL and measuring its thickness are perhaps the most meaningful functions that can be performed with these technologies because the true health of the optic nerve is more accurately reflected in the nerve fiber layer than in the structure of the optic disc. All devices provide estimates of peripapillary RNFL thickness. The HRT bases its estimate on relative height of the inner limiting membrane. Of course, there can be other reasons for focal changes in retinal elevation, and these can create artifacts in the data. Also, retinal elevation is referenced to a point temporal to the optic disc. The stability of that reference point over time has also been questioned, and it may definitely change in pathologic conditions that do not involve changes in nerve fiber layer thickness at that point. OCT provides a representation of the actual thickness of the RNFL. However, conditions such as retinal edema or swelling can alter the apparent thickness of the RNFL without any change in the number of axon bundles. If care is taken to recognize these conditions, OCT measurements reflect focal thinning and loss of nerve fiber. Studies comparing changes in RNFL thickness with visual field progression are not yet available for OCT. The GDx is designed to quickly and accurately estimate RNFL thickness and to monitor changes over time, but it lacks the versatility of the other two instruments. In addition, the frequent upgrading of the technology has limited the number of longitudinal studies that have been performed using this instrument, and, therefore, its usefulness in quantifying RNFL loss over time and comparing progression of RNFL loss with progression of visual field defects has not been established. At present, both the OCT and GDx are manufactured by Carl Zeiss Meditec. Therefore, the future marketing strategy for these previously competing instruments is uncertain. Because the GDx has limited use, it has not been favored by multispecialty practices. It is our opinion that, of the other two instruments, the HRT is more useful for evaluating optic disc swelling and elevation. The OCT appears to be most convenient and accurate for determining and following RNFL thickness and has become the instrument most preferred for assessment of macular disease. Practitioners may also wish to consider cost, simplicity of use, versatility and permanence of the technology, and technical support provided. No one instrument is optimal for all factors. The GDx is most economical but its use is most limited and its future in ophthalmology is less certain. The greatest amount of data from published studies are available for the HRT, and the technology appears to have survived several upgrades and remains compatible over versions. However, it does not produce the quality cross- sectional diagnostic images of RNFL and macula that the OCT can now provide. Yet for its apparent usefulness, the OCT does not have the track record of the HRT, nor has it be shown to be useful in monitoring changes in optic disc swelling. Computerized imaging technologies provide a useful and desirable adjunct to the standard clinical examination, in the manner that computerized threshold perimetry has provided quantitative data and a more objective, reproducible assessment of visual fields. But genuine defects can almost always be detected qualitatively by careful clinical 293 J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 Trick et al examination. Moreover, clinical correlation is mandatory to confirm and verify the abnormalities signaled by the imaging devices, as it is for automated perimetry. Once these correlations are established for a given patient, these imaging devices are useful to monitor for changes that may be more difficult to detect by other clinical methods. Because the measurement reproducibility of the three technologies is high, each holds promise for improving detection of optic nerve damage and its progression. The important role these devices play in the management of patients with various retinopathies and with glaucomatous optic neuropathy is now well established. Research studies designed to evaluate the three different imaging technologies in non- glaucomatous optic nerve diseases are needed to provide relevant data concerning their usefulness in papilledema and other optic neuropathies. The marketplace has to determine which instrument will dominate, but it is certain that computerized analysis of optic disc and RNFL images in optic nerve disease will be an integral component of clinical practice. REFERENCES 1. Weinreb RN, Lusky M, Bartsch D, et al. Effect of repetitive imaging on topographic measurements of the optic nerve head. Arch Ophthalmol 1993; 111: 636- 8. 2. Hatch Wy Trope GE, Buys YM, et al. Agreement in assessing glaucomatous discs in a clinical teaching setting with stereoscopic disc photographs, planimetry, and laser scanning tomography. J Glaucoma 1999; 8: 99- 104. 3. Ervin JC, Lemij HG, Mills RP, et al. Clinician change detection viewing longitudinal stereophotographs compared to confocal scanning laser tomography in the LSU Experimental Glaucoma ( LEG) Study. Ophthalmology 2002; 109: 467- 81. 4. Chauhan BC, McCormick TA, Nicolela MT, et al. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol 2001; 119: 1492- 9. 5. Knighton RW, Huang XR. Linear birefringence of the central human cornea. Invest Ophthalmol Vis Sci 2002; 43: 82- 6. 6. Greenfield DS, Knighton RW, Huang XR. Effect of corneal polarization axis on assessment of retinal nerve fiber layer thickness by scanning laser polarimetry. Am J Ophthalmol 2000; 129: 715- 22. 7. Greenfield DS, Knighton RW, Feuer WJ, et al. Correction for corneal polarization axis improves the discriminating power of scanning laser polarimetry. Am J Ophthalmol 2002; 134: 27- 33. 8. Sanchez- Galeana C, Bowd C, Blumenthal EZ, et al. Using optical imaging summary data to detect glaucoma. Ophthalmology 2001; 108: 1812- 8. 9. Greaney MJ, Hoffman DC, Garway- Heath DF, et al. Comparison of optic nerve imaging methods to distinguish normal eyes from those with glaucoma. Invest Ophthalmol Vis Sci 2002; 43140- 5. 10. ZangwillL, Iraki, Berry CC, etal. Effect of cataract and pupil size on image quality with confocal scanning laser ophthalmoscopy. Arch Ophthalmol 1997; 115: 983- 90. 11. Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci 2004; 45: 1716- 24. 12. Chauhan BC, Blanchard JW, Hamilton DC, et al. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using laser tomography. Invest Ophthalmol Vis Sci 2000; 41: 775- 82. 13. Salgarello T, Falsini B, Tedesco S, et al. Correlation of optic nerve head tomography with visual field sensitivity in papilledema. Invest Ophthalmol Vis Sci 2001; 42: 1487- 94. 14. Trick GL, Bhatt SS, Dahl D, et al. Optic disc topography in pseudopapilledema: a comparison to pseudotumor cerebri. J Neuro-ophthalmol 2001; 21: 240^ k 15. Gobel W, Lieb WE, Grein HI Quantitative and objective follow- up of papilledema with the Heidelberg Retina Tomograph ( in German). Ophthalmologe 1997; 94: 673- 7. 16. Hoye VJ 3rd, Berrocal AM, Hedges TR 3rd, et al. Optical coherence tomography demonstrates subretinal macular edema from papilledema. Arch Ophthalmol 2001; 119: 1287- 90. 17. Mashima Y, Kimura I, Yamamoto Y, et al. Optic disc excavation in the atrophic stage of Leber's hereditary optic neuropathy: comparison with normal tension glaucoma. Graefes Arch Clin Exp Ophthalmol 2003; 241: 75- 80. 18. Floyd MS, Katz BJ, Digre KB. Measurement of the scleral canal using optical coherence tomography in patients with optic nerve drusen. Am J Ophthalmol 2005; 139: 664- 9. 19. Lefrancois A, Barrault O, Pare C, et al. Optic disk drusen: what are the advantages of the new imaging techniques? ( in French). J Fr Ophtalmol 2003; 26: S23- 30. 20. Ocakoglu O, Ustundag C, Koyluoglu N, et al. Long term follow- up of retinal nerve fiber layer thickness in eyes with optic nerve head drusen. Curr Eye Res 2003; 26: 277- 80. 21. Roh S, Noecker RJ, Schuman JS, et al. Effect of optic nerve head drusen on nerve fiber layer thickness. Ophthalmology 1998; 105: 878- 85. 22. Moussalli MA, Sanseau A, Ebner R. Papillary drusen and ocular hypertension. Int Ophthalmol 2001; 23: 275- 8. 23. Tatlipinar S, Kadayifcilar S, Bozkurt B, et al. Polarimetric nerve fiber analysis in patients with visible optic nerve head drusen. J Neuroophthalmol 2001; 21: 245- 9. 24. Kuchenbecker J, Wecke T, Vorwerk CK, et al. Quantitative and objective topometrical analysis of drusen of the optic nerve head with the Heidelberg retina tomograph ( HRT) ( in German). Ophthalmologe 2002; 99: 768- 73. 25. Foroozan R, Buono LM, Savino PJ, et al. Scanning laser polarimetry of the retinal nerve fiber layer in central retinal artery occlusion. Ophthalmology 2003; 110: 715- 8. 26. Colen TP, van Everdingen JA, Lemij HG. Axonal loss in a patient with anterior ischemic optic neuropathy as measured with scanning laser polarimetry. Am J Ophthalmol 2000; 130: 847- 50. 27. Kuprjanowicz L, Goslawski W, Karczewicz D, et al. Evaluation of retinal nerve fiber thickness with scanning laser polarimetry in patients with anterior ischemic optic neuropathy ( in Polish). Klin Oczna 2004; 106( 3 Suppl): 440- 2. 28. Danesh- Meyer H, Savino PJ, Spaeth GL, et al. Comparison of arteritis and nonarteritic anterior ischemic optic neuropathies with the Heidelberg Retina Tomograph. Ophthalmology 2005; 112: 1104- 12. 29. Meier FM, Bernasconi P, Sturmer J, et al. Axonal loss from acute optic neuropathy documented by scanning laser polarimetry. Br J Ophthalmol 2002; 86: 285- 7. 30. Steel DH, Waldock A. Measurement of the retinal nerve fibre layer with scanning laser polarimetry in patients with previous demyelin-ating optic neuritis. J Neurol Neurosurg Psychiatry 1998; 64: 505- 9. 31. Parisi V, Manni G, Spadaro M, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 1999; 40: 2520- 7. 32. Parisi V Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer's disease. Semin Ophthalmol 2003; 18: 50- 7. 33. Monteiro ML, Medeiros FA, Ostroscki MR. Quantitative analysis of axonal loss in band atrophy of the optic nerve using scanning laser polarimetry. Br J Ophthalmol 2003; 87: 32- 7. 294 © 2006 Lippincott Williams & Wilkins Imaging of Optic Disc and Retina J Neuro- Ophthalmol, Vol. 26, No. 4, 2006 34. Banks MC, Robe- Collignon NJ, Rizzo JF 3rd, et al. Scanning laser polarimetry of edematous and atrophic optic nerve heads. Arch Ophthalmol 2003; 121: 484- 90. 35. Kanamori A, Nakamura M, Matsui N, et al. Optical coherence tomography detects characteristic retinal nerve fiber layer thickness corresponding to band atrophy of the optic discs. Ophthalmology 2004; 111: 2278- 83. 36. Tatsumi Y, Kanamori A, Kusuhara A, et al. Retinal nerve fiber layer thickness in optic tract syndrome. Jpn J Ophthalmol 2005; 49: 294- 6. 37. Miyahara T, Kurimoto Y, Kurokawa T, et al. Alterations in retinal nerve fiber layer thickness following indirect traumatic optic neuropathy detected by nerve fiber analyzer, GDx- N. Am J Ophthalmol 2003; 136: 361^. 38. Medeiros FA, Moura FC, Vessani RM, et al. Axonal loss after traumatic optic neuropathy documented by optical coherence tomography. Am J Ophthalmol 2003; 135: 406- 8. 39. Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber's hereditary optic neuropathy. Ophthalmology 2005; 112: 120- 6. 40. Savini G, Barboni P, Valentino ML. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber's hereditary optic neuropathy mutations. Ophthalmology 2005; 112: 127- 31. 41. Zoumalan CI, Agarwal M, Sadun AA. Optical coherence tomography can measure axonal loss in patients with ethambutol- induced optic neuropathy. Graefes Arch Clin Exp Ophthalmol 2005 ; 243: 410- 6. 42. Viestenz A, Viestenz A, Mardin CY Vigabatrin- associated bilateral simple optic nerve atrophy with visual field constriction: a case report and a survey of the literature ( in German). Ophthalmologe 2003; 100: 402- 5. 43. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology 1995; 102: 748- 56. 44. Zambarakji HJ, Schlottmann P, Tanner V, et al. Macular microholes: pathogenesis and natural history. Br J Ophthalmol 2005; 89: 189- 93. 45. Duker JS, Puliafito CA, Wilkins JR, et al. Imaging fellow eyes in patients diagnosed with idiopathic macular holes using optical coherence tomography. In: Final Program of the Annual Meeting of the American Academy of Ophthalmology ( AAO), 1995, Oct 29- Nov 3. Atlanta, GA: AAO; 1995: 118. 46. Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol 2004; 138: 732- 9. 47. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology 1995; 102: 217- 29. 48. Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol 1995; 113: 1019- 20. 49. Tewari HK, Sony P, Chawla R, et al. Prospective evaluation of intravitreal triamcinolone acetonide injection in macular edema associated with retinal vascular disorders. Eur J Ophthalmol 2005; 15: 619- 26. 50. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of central serous chorioretinopathy. Am J Ophthalmol 1995; 120: 65- 74. 51. Montero JA, Ruiz- Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol 2005; 89: 562^ k 52. Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age- related macular degeneration and choroidal neovascularization. Ophthalmology 1996; 103: 1260- 7. 53. Rogers AH, Martidis A, Greenberg PB, et al. Optical coherence tomography findings following photodynamic therapy of choroidal neovascularization. Am J Ophthalmol 2002; 134: 566- 7. 54. Rich RM, Rosenfeld PJ, Puliafito CA. Short- term safety and efficacy of intravitreal bevacizumab ( Avastin) for neovascular age- related macular degeneration. Retina 2006; 26: 495- 511. 55. Tong L, Ang A, Vernon SA, et al. Sensitivity and specificity of a new scoring system for diabetic macular oedema detection using a con-focal laser imaging system. Br J Ophthalmol 2001; 85: 34- 9. 56. Zambarakji HJ, Butler TK, Vernon SA. Assessment of the Heidelberg retina tomograph in the detection of sight- threatening diabetic mac-ulopathy. Eye 1999; 13: 136^ 4. 57. Guan K, Hudson C, Flanagan JG. Comparison of Heidelberg retina tomograph II and retinal thickness analyzer in the assessment of diabetic macular edema. Invest Ophthalmol Vis Sci 2004; 45: 610- 6. 295 |