| OCR Text |
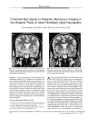
Show ORIGINAL CONTRIBUTION A Functional Magnetic Resonance Imaging Study in Patients with Benign Essential Blepharospasm Robert S. Baker, MD, Anders H. Andersen, PhD, Robert J. Morecraft, PhD, and Charles D. Smith, MD Objective: To identify blinking- induced functional magnetic resonance imaging ( fMRI) activation patterns in five benign essential blepharospasm ( BEB) patients and five age- matched control subjects. Methods: fMRI brain activation maps were obtained during repeated conditions of spontaneous and voluntary blinking in BEB and control groups. Blood oxygen level-dependent intensity images were collected from two separate runs as 16 axial and 16 coronal, 8 mm thick slices using a T2- star weighted gradient echo EPI sequence, coregis-tered with anatomic images. Spatially normalized and iso-tropically blurred activation maps for each subject were combined within groups of BEB patients and control subjects to generate maps of the intersubject mean fractional signal change. Results: Substantially greater activation during spontaneous and voluntary blinking was seen in BEB patients compared with control subjects in the anterior visual cortex, anterior cingulate cortex, primary motor cortex, central region of the thalamus, and superior cerebellum. In both groups, activations were generally greater for voluntary than for spontaneous blinking. Conclusions: The activations observed might represent a hyperactive cortical circuit linking visual cortex, limbic system, supplementary motor cortex, cerebellum, and supranuclear motor pathways innervating the periorbital muscles. ( JNeuro- Ophthalmol 2003; 23: 11- 15) From the Departments of Ophthalmology ( RSB), Neurology ( CDS), and Anatomy & Neurobiology ( AHA, CDS), University of Kentucky College of Medicine & Magnetic Resonance Imaging and Spectroscopy Center, Lexington, Kentucky, and the Division of Basic Biomedical Sciences, University of South Dakota School of Medicine, Vermillion, South Dakota ( RJC). Address correspondence to Robert S. Baker, MD, East 304 Kentucky Clinic, Lexington, KY 40436- 0284, USA; E- mail: rsbmail@ pop. uky. edu The neural substrate of benign essential blepharospasm ( BEB) is unknown. We have applied functional magnetic resonance imaging ( fMRI) to map cortical and subcortical activations during blinking in normal adults and in patients with BEB to help elucidate this substrate. A previous functional imaging study using positron emission tomography demonstrated increased metabolism in the thalamus and striatum in BEB patients ( 1). Our hypothesis is that BEB involves cortical as well as subcortical circuit abnormalities, and that fMRI mapping might better elucidate potential cortical components. We present evidence that BEB is associated with cortical activation differences in cingulate, primary motor, and anterior visual cortices, and in the cerebellum. Our data also confirm thalamic and striatal differences reported previously. MATERIALS AND METHODS Participants Ten individuals were analyzed in this study: five normal volunteers and five patients from the Facial Movement Disorders Clinic at the University of Kentucky. Normal volunteers were two men and three women ranging in age from 50 to 62 years. Patients were four women and one man in the same age range, all diagnosed with BEB and receiving botulinum toxin injections, but not taking psychotropic drugs. All patients were mildly symptomatic at the time of the testing but were able to adequately perform the protocol; that is, they could generate voluntary blinks without spasms, keeping the eyes closed for longer than a few hundred milliseconds, and could maintain eye closure without noticeable lower facial movement. None of the patients had Meige syndrome. All participants were rehearsed in generating voluntary blinks at a rate of 80 to 100 per minute. Subjects were instructed to maintain this pace in each voluntary epoch in the scanner without actually counting blinks. An auditory cue was used to cause subjects to switch task. Functional Activation Paradigm Subjects alternated between three task states in a blocked experimental design: 40 seconds with eyes closed, Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. J Neuro- Ophthalmol, Vol. 23, No. 1, 2003 11 JNeuro- Ophthalmol, Vol. 23, No. 1, 2003 Baker et al. 40 seconds with eyes open ( spontaneous blinking), and 40 seconds of self- paced voluntary blinking ( one to two blinks per second). This sequence was repeated in order four times throughout the imaging time period. The protocol was performed in darkness. No light source or visual fixation was used. Protocol compliance was assessed by pre- scan training and by post- scan interview, but there was no in- magnet measurement of blink rate or duration. Image Acquisition Functional magnetic resonance images were collected in the Magnetic Imaging and Spectroscopy Center ( MRISC) on a standard Siemens Magnetom VISION 1.5 Tesla imaging system, using a circularly polarized transmit/ receive head coil. Foam padding was used to stabilize head position. Blood oxygen level- dependent signal intensity data were collected from two separate runs as 16 axial and 16 coronal, 8 mm thick slices covering the entire cerebrum and upper cerebellum and brainstem. Owing to the unavoidable presence of magnetic field susceptibility artifacts, activation in some areas of the brain are best imaged in axial slices, whereas other areas, for example, basal ganglia and thalamus, are better imaged at a coronal orientation. In the axial acquisition, the slice orientation was slightly oblique ( toward the coronal view) to avoid image reconstruction artifacts from eye movements. For each orientation, a T2- star weighted gradient echo EPI sequence with minimal inflow weighting was used with these acquisition parameters: TR/ TE = 4000/ 45 milliseconds, 90 degrees flip angle, matrix = 128 x 128, field of vision = 256 x 256 mm. A total of 128 EPI image volumes were acquired during each run. fMRI data were acquired during two separate runs, one at axial and the other at coronal orientations. The images were motion corrected in data post processing with SPM' 99 ( 2) and Matlab software using computing facilities in the MRISC. There were no systematic differences in the pattern or extent of motion artifacts between the groups, and no subject data were excluded from the subsequent analysis due to excessive movement. A three-dimensional MPRAGE sequence ( TR/ TE/ TI = 11.4 msec/ 4.4 msec/ 300 msec, FA = 8 degrees, slice thickness = 2 mm) was used to collect anatomic images for the localization of functional activity and for the registration of fMRI data sets to the stereotactic space of Talairach and Tournoux ( 3). The transformation to stereotaxic space was done using AFNI software ( 5). An anatomic reference image consisting of the mean of the intensity- normalized MPRAGE images from all subjects in each group was used to display group mean activation maps. Data Analysis The fMRI data were analyzed with AFNI software using the cross- correlation method, or equivalently, an independent samples t test ( 4,5). For each subject and for each pairing of task states, correlation coefficients were computed voxel by voxel using a boxcar reference waveform with no time lag. The first image of each run was discarded due to the approach of the MRI signal to a steady state. The resulting functional parametric maps contain a value for the mean fractional signal change ( AS/ S) and an associated correlation coefficient ( r value) at each voxel. These maps, referenced to each subj ect' s own anatomic images, were transformed to a common stereotactic Talairach coordinate frame and resampled at 2 mm x 2 mm x 2 mm resolution using cubic spline interpolation ( 3). Spatially normalized and isotropically blurred ( Gaussian kernel, CT = 2 mm) activation maps for each subject were combined within groups of BEB patients and normal control subjects to generate maps of the intersubject mean fractional signal changes and associated mean r values ( 5). Data from both the coronal and axial orientations were used. A standard r-to z- conversion was used to obtain the equivalent z- score for the group average activation map at each voxel location ( 6). RESULTS Spontaneous Blinks versus Eyes Closed We observed decreased activation bilaterally in the occipital and parietal association regions during spontaneous blinking compared with the eyes- closed state, but only in control subjects. In contrast to these results, we observed extensive increased activation of the primary visual cortex, area prostriata, and occipital visual association areas in BEB patients during blinking, but no areas of deactivation ( Fig. 1A, Table 1). Voluntary Blinks versus Eyes Closed Control subjects showed bilaterally increased activation in primary visual cortex, central thalamus, posterior putamen, and supplementary and primary motor cortex in this task comparison. These same areas activated in the BEB patients, but to a greater extent. The BEB patients demonstrated activation further anteriorly in the calcarine cortex ( putative area prostriata) than control subjects. Control subjects activated the vermis and left hemisphere of the cerebellum. BEB patients demonstrated more extensive increased activation in this region, involving larger portions of the vermis and both superior lobes of the cerebellum. An area of activation in the cingulate cortex, corresponding to the rostral cingulate motor area ( M3), which is known to receive direct projections from area prostriata, was present in BEB ( Fig. IB, Table 2). Voluntary versus Spontaneous Blinks Both BEB and control groups exhibited increased activation in primary visual cortex, primary motor cortex, cerebellar paravermian area, central thalamus, and anterior Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 12 © 2003 Lippincott Williams & Wilkins JMRI IN BLEPHAROSPASM JNeuro- Ophthalmol, Vol. 23, No. 1, 2003 FIG. 1. A: Activation maps in the spontaneous blinking versus eyes- closed contrast generated at the same threshold z- score of 4.5, superimposed on averaged anatomic images in Talairach coordinates. Data from control participants are shown in the top row, from benign essential blepharospasm ( BEB) patients in the bottom row. Activation is decreased ( blue) in the visual association cortex and minimally increased ( red- yellow spectrum) in the primary visual cortex in control subjects. There is markedly increased activation in the primary visual cortex in BEB patients. B: Activation maps in the voluntary blinking versus eyes- closed contrast. Images demonstrate increased activation in BEB patients in the cerebellar hemispheres bilaterally, the cerebellar vermis, anterior visual cortex ( short arrow), left inferior occipital cortex, posterior putamen bilaterally, the central thalamus bilaterally, and the left anterior cingulate gyrus ( asterisk). C: Activation maps in the voluntary versus spontaneous blinking contrast. ( Talairach z- coordinate given at bottom of figure). Images demonstrate increased activation in BEB patients in the cerebellar hemispheres bilaterally, the cerebellar vermis, primary motor cortex bilaterally ( arrowheads), anterior visual cortex, left inferior occipital cortex, posterior putamen bilaterally, central thalamus bilaterally, and left anterior cingulate gyrus. cingulate gyrus. The activations in these regions appeared stronger in control subjects ( Table 3) with this task contrast. DISCUSSION The main result of this study was demonstration of fMRI activation of several cortical and subcortical regions normally engaged in simple blinking, and increased activation in many of these regions during blinking in patients with BEB. In voluntary blinking, these regions are primary visual cortex and area prostriata, primary motor cortex, cingulate cortex, posterior putamen, central thalamus, and the superior cerebellar hemispheres and vermis. Bilateral activation was the rule, and we found little evidence for a right hemisphere localization of blinking in our data. However, the correlational technique used in our image analysis does not allow us to address the issue of cerebral dominance for blinking ( 7,8). There are several caveats that should be observed in the interpretation of our results. Susceptibility artifacts in the medial inferior frontal region preclude any conclusion regarding the presence or absence of activations in this area, including the caudate head, midline anterior frontal, and Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 13 JNeuro- Ophthalmol, Vol. 23, No. 1, 2003 Baker et al. were not able to observe in the magnet whether some of the blinks in the BEB subjects may have been associated with spasms. However, as noted above, we observed all participants in a training session and all were able to generate voluntary blinks at the desired frequency. It nonetheless remains possible that BEB patients had more difficulty achieving this frequency than control subjects and that some group differences would have been smaller at lower voluntary frequencies. Further important refinements in technique to address each of these potential confounds are underway in our laboratory and will be the subject of subsequent reports. Cortical innervation of the facial motor nucleus and the interplay between limbic systems and neocortex could be involved in blepharospasm. Including these cortical components in a model for blepharospasm could lead to an improved understanding of BEB. Multiple cortical areas controlling facial musculature in the human have homologous counterparts in the monkey ( 9- 11). Recently, More-craft et al ( 12) demonstrated that somatotopically organized face areas in supplementary motor cortex ( M2), rostral cin-gulate motor cortex ( M3), and caudal cingulate motor cortex ( M4) project directly to the facial nucleus. A direct connection exists between area M3 in the rostral cingulate motor cortex and an architectonically distinct region ( area prostriata) bordered by the primary visual cortex posteriorly and the limbic cortex anteriorly ( 12). This connection establishes a more direct linkage between cortex bordering the primary visual region and corticofacial projections than the classic visuomotor pathway from lateral pari-eto- occipital cortex to prefrontal cortex and from parietal lobe to rostrolateral premotor cortex. The current study TABLE 2. Comparison of locations of functional magnetic resonance imaging ( fMRI) activations in the voluntary blinking and eyes closed conditions in darkness Location Primary visual B SMAB Cingulate gyrus L Cingulate gyrus L Primary motor Posterior putamen B Centromedian thalamus B Red nucleus B Cerebellar vermis B Cerebellar hemisphere B Talairach coordinates 2,- 74,13 3, - 8 , 57 - 9, 0, 37 - 11,- 14,35 48, - 4, 48 28, - 17, 4 9, - 17, 8 9, - 22, - 3 3,- 61,- 20 29, - 65, - 25 Brodmann area BA17 BA6 BA24 BA 23/ 24 BA4 - - - - - z- score 7.8 6.1 2.5 ns 6.0 4.4 6.5 2.1 4.5 6.3 ( left only) z- BEB 11.0 7.7 6.6 6.8 6.6 7.7 7.9 6.3 8.9 10.9* Z- scores are given when a region demonstrated a significantly increased (+) or decreased (-) activation in this task comparison. For bilateral activations, the z- score and location for the greater of the two peak activations is listed. All activations are positive ( increased). * Bilateral but greater extent observed on L. B, bilateral; BEB, benign essential blepharospasm; L, left; ns, not significant; R, right. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 14 © 2003 Lippincott Williams & Wilkins TABLE 1. Comparison of locations of functional magnetic resonance imaging ( fMRI) activations in the spontaneous blinking and eyes closed conditions in darkness Location Primary visual B Primary visual B Inferior occipital B Inferior occipital B Superior parietal B Talairach coordinates 3,- 75, 10 3, - 65, 7 23, - 66, - 12 43,- 63,- 15 38, - 44, 44 Brodmann area BA17 BA17 BA19 BA19 BA7 z- score + 4.5 ns - 4.9 - 4.7 - 4.7 z- BEB + 9.8 + 10.1 + 5.6 ns ns Z- scores are given when a region demonstrated a significantly increased (+) or decreased (-) activation in this task comparison. For bilateral activations, the z- score and location for the greater of the two peak activations is listed. B, bilateral; BEB, benign essential blepharospasm; L, left; ns, not significant; R, right. medial orbitofrontal cortex. Our use of a blocked experimental design did not allow us to generate a reference function based on the series of blinking events generated by each subject, likely limiting the sensitivity of fMRI detection, particularly in the control subjects. Finally, because we did not monitor blinking while subjects were being scanned, we cannot exclude potential differences in blink rate or duration between the BEB and control groups. We fMRI IN BLEPHAROSPASM JNeuro- Ophthalmol, Vol. 23, No. 1, 2003 TABLE 3. Comparison of locations of functional magnetic resonance imaging ( fMRI) activations in the voluntary blinking and spontaneous blinking conditions in darkness Location Talairach coordinates Brodmann area BA17 BA24 BA4 BA19 BA19 - - - - z- score 6.6 4.3 5.4 6.0 ns 3.8 4.5 4.2 6.4 z- BE ns 5.7 6.4 9.0 7.8 6.2 6.2 7.3 7.8' Primary visual B Cingulate gyrus L Primary motor Inferior occipital L Inferior occipital L Posterior putamen B Centromedian thalamus B Cerebellar vermis Cerebellar hemisphere B ± 2, - 70, 17 - 9 , 8, 37 ± 48, - 4 , 48 - 23, - 6 3 , - 17 - 25, - 80, 8 ± 28, - 17, 4 ± 11,- 17,8 ± 3, - 6 1 , - 20 ± 29, - 65, - 25 Z- scores are given when a region demonstrated a significantly increased (+) or decreased (-) activation in this task comparison. For bilateral activations, the z- score and location for the greater of the two peak activations is listed. All activations are positive ( increased). * Bilateral but greater extent observed on L. B, bilateral; BEB, benign essential blepharospasm; L, left; ns, not significant; R, right. demonstrates enhanced activation of both area prostriata and anterior cingulate cortex in patients with BEB making spontaneous and voluntary blinks. Therefore, this pathway may be important in mediating facial motor responses to visual stimuli. Our observations suggest that the cortical components of this pathway may be excessively active in BEB, unlike the primary somatosensory components, which may be less active ( 13). We consider these results preliminary but important to future efforts to understand BEB. REFERENCES 1. Esmaeli- Gutstein B, Nahmias C, Thompson M, et al. Positron emission tomography in patients with benign essential blepharospasm. Ophthal Plast Recon Surg 1999; 15: 23- 7. 2. Friston KJ, Ashburner J, Poline JB, et al. Spatial registration and normalization of images. Human Brain Mapp 1995; 2: 165- 89. 3. Talairach J, Tournoux P. Co- Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, 1988. 4. Bandettini PA, Jesmanowicz A, Wong EC, et al. Processing strategies for time- course data sets in functional MRI of the human brain. Magn Reson Med 1993; 30: 161- 73. 10. 11. 12. 13. Cox RW. AFNI: software for analysis and visualization of neuroim-ages. ComputBiomedRes 1996; 29: 162- 73. Smith CD, Andersen AH, Kryscio RJ, et al. Differences in functional magnetic resonance imaging activation by category in a visual confrontation naming task. J Neuroimaging 2001 ; 11: 165- 70. van Eimeren T, Boecker H, Konkiewitz EC, et al. Right lateralized motor cortex activation during volitional blinking. Ann Neurol 2001; 49: 813- 6. Tsubota K, et al. Functional MRI brain activation by eye blinking. Exp Eye Res 1999; 59: 1- 7. Penfield W, Welch K. Supplementary motor area of the cerebral cortex: a clinical and experimental study. Arch Neurol Psychiatr 1951; 66: 289- 317. Muakkassa KF, Strick AL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized premotor areas. Brain Res 1979; 177: 176- 82. Woolsey CN, Settlage PH, Meyer DR, et al. Patterns of localization in precentral and supplementary motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis 1952; 30: 238- 64. Morecraft RJ, Rockland KS, Van Hoesen GW. Localization of area prostriata and its projection to the cingulate motor cortex in the Rhesus monkey. Cereb Cortex 2000; 10: 197- 203. Feiwell RJ, Black KJ, McGee- Minnich LA, et al. Diminished regional cerebral blood flow response to vibration in patients with blepharospasm. Neurology 1999; 52: 291- 7. Copyright © Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. 15 |