| OCR Text |
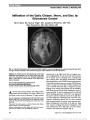
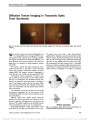
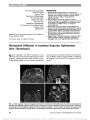
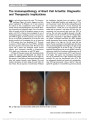
Show Downbeat Nystagmus Secondary to Familial Hemophagocytic Lymphohistiocytosis Cindy X. Cai, BS, Frank S. Siringo, MD, OD, Jeffrey G. Odel, MD, Angela Lignelli-Dipple, MD, Bryan A. Lanzman, MD, Tatyana Gindin, MD, Alexandra H. Filipovich, MD Abstract: Hemophagocytic lymphohistiocytosis is a rare autosomal recessive disorder characterized by severe inflammation induced by defective natural killer cell func-tion, which triggers a state of highly stimulated but ineffective immune response. This disorder can affect multiple organ systems, and neurologic manifestations include irritability, seizures, impaired consciousness, men-ingismus, and cranial nerve palsies. We describe a unique case of hemophagocytic lymphohistiocytosis in which down-beat nystagmus developed due to cerebellar swelling with compression of the cervicomedullary junction. Journal of Neuro-Ophthalmology 2014;34:57-60 doi: 10.1097/WNO.0000000000000064 © 2013 by North American Neuro-Ophthalmology Society Hemophagocytic lymphohistiocytosis (HLH), which may be familial or acquired, is a severe inflammatory disorder caused by disruption of T-cell function that triggers a state of hypercytokinemia, resulting in a highly stimulated but ineffective immune response. Downbeat nystagmus (DBN) is an uncommon disorder of ocular motility that typically localizes to the posterior fossa, particularly the cervicomedullary junction. We are unaware of previous re-ports of DBN occurring in the setting of HLH. CASE REPORT A previously healthy ex-full-term 10-month-old dizygotic twin girl was brought by her parents to the emergency department for second opinion after suffering 2 months of intermittent fevers to 40.5°C, diarrhea, and progressive abdominal distension. Previously, she had been hospitalized for intensive supportive care including empiric intravenous ceftazidime and gentamicin. Her course had been further complicated by hepatosplenomegaly, lymphadenopathy, and disseminated intravascular coagulation. Because of new-onset strabismus, the ophthalmology service was consulted. On examination, the patient was hypotonic with open and flat anterior fontanelles. She could fix and follow with each eye but had a marked abduction deficit with saccadic slowing of the left eye. Pupils were equal and reactive without a relative afferent defect. The anterior and posterior segments were normal. The examination was most notable for prominent primary position downbeat nystagmus (DBN). Laboratory testing demonstrated a white blood cell count of 5,400/cm3 (normal: 6,000-17,500/cm3), hemo-globin of 8.5 g/dL (normal: 9.5-13.5 g/dL), platelet count of 134,000/cm3 (normal: 165,000-415,000/cm3), mild hyponatremia, and normal hepatic function tests. Erythrocyte sedimentation rate was 120 mm/hr, triglycerides 1,065 mg/dL (normal: 30-200 mg/dL), and fibrinogen 151 mg/dL (normal: 99-466 mg/dL). Opening pressure on lumbar puncture was normal, and cerebrospinal fluid (CSF) analysis showed a poly-clonal, predominantly T-cell population highlighted with CD45 gating. Cultures for bacteria and fungus and parasitol-ogy studies were negative; polymerase chain reaction for cytomegalovirus, Epstein-Barr virus and Parvovirus B19 was negative. Further testing revealed increased ferritin of 2,284 ng/mL (normal: 10-150 ng/mL), increased mean chan-nel fluorescence of perforin in natural killer (NK) cells, absent NK cell function, cell function, decreased NKT cells, increased CD8 T lymphocytes (26%; normal: 0%-4%), and increased slL-2 receptor (CD25) (.3000; normal: 0-1033). Magnetic resonance imaging (MRI) of the brain showed diffuse cerebral atrophy with mild increase in cerebellar volume (Fig. 1A) and leptomeningeal enhancement of the cerebellum (Fig. 1B). The cerebellar tonsils were low- Columbia University College of Physicians and Surgeons (CXC), New York, New York; Departments of Ophthalmology (FSS, JGO), Radiology (AL), and Pathology (TG); Columbia University Medical Center, New York Presbyterian Hospital, New York, New York; and Department of Clinical Immunology (AHF), Cincinnati Children's Hospital, University of Cincinnati, Cincinnati, Ohio. The authors report no conflicts of interest. Address correspondence to Cindy X. Cai, BS, 630 W 168th Street, Mailbox #347, New York, NY 10032; E-mail: cxc2103@columbia.edu Cai et al: J Neuro-Ophthalmol 2014; 34: 57-60 57 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. lying and crowded with decreased CSF space at the cervi-comedullary junction, but no Arnold-Chiari malformation was noted. Bone marrow aspirate demonstrated hemophagocytosis (Fig. 2A) with increased bone marrow macrophages (Fig. 2B). Results of flow cytometry from the bone marrow were similar to those obtained from the CSF. Genetic testing revealed a mutation in the STXBP2 gene with normal UNC13D. There was no known history of consanguinity in the parents. The patient was treated with etoposide and dexameth-asone, and, after 48 hours, there was improvement in mental status with resolution of DBN and the left sixth nerve palsy. Follow-up MRI revealed decreased cerebellar swelling (Fig. 3). The patient received an allogenic hema-topoietic stem cell transplant (HSCT) 6 months after the initial presentation and had 90% engraftment with normal antibody titers 18 months later. DISCUSSION Hemophagocytic lymphohistiocytosis (HLH) is part of a heterogeneous group of disorders initially described in 1952 by Farquhar and Claireaux (1). The frequency of this disorder is difficult to assess but estimated as 1 in 50,000- 100,000 live births (2,3). On the molecular level, familial forms of the disorder often are caused by defects in perforin or other proteins involved in granule-dependent cytotoxicity (4-7). NK and cytotoxic T lymphocytes normally store per-forin and granzyme proteins in specialized secretory lysosomes that are released upon encountering a target cell, leading to target cell apoptosis (8). When this mechanism is dysfunc-tional, there is an abnormal and excessive production of T-cell-derived cytokines ("cytokine storm") leading to uncon-trolled accumulation of activated T lymphocytes and activated histiocytes (macrophages) in various tissues, resulting in hemophagocytosis and organ damage (8-10). Genetic defects in familial hemophagocytic lymphohis-tiocytosis (fHLH) are autosomal recessive and continue to expand. Currently, 5 genetic loci (FHL 1-5) have been identified (9). FHL1 encodes an as-yet unidentified protein. FHL2 accounts for up to one-third of fHLH cases because of defects in PRF1 (perforin), a cytolytic protein used by NK and cytotoxic T lymphocytes. Perforin polarizes to the plasma membrane of target cells, delivering cytotoxic enzymes (granzymes) to the target cell, inducing apoptosis. FIG. 1. A. Precontrast T1 sagittal magnetic resonance imaging shows diffuse cortical atrophy, cerebellar swelling with low-lying tonsils. B. Postcontrast T1 axial magnetic resonance imaging reveals cerebellar leptomeningeal enhancement. FIG. 2. Bone marrow aspirate. A. There are abundant macrophages with soft granular cytoplasm and evidence of eryth-rocytes undergoing phagocytosis (arrows) (hematoxylin and eosin, ·400). B. CD68 immunostaining demonstrates numerous macrophages (·400). 58 Cai et al: J Neuro-Ophthalmol 2014; 34: 57-60 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Other genetic loci in fHLH encode proteins facilitating delivery of perforin to target cells, including a priming fac-tor munc13-4 (UNC13D), and membrane fusion proteins syntaxin 11 (STX11) and munc18-2 (STXBP2). The clinical syndrome of HLH also can be trigged by infection, autoimmune disease, and malignancy, and are designated acquired HLH (7,11). Macrophage activation syn-drome typically is used to designate HLH due to autoimmune diseases, particularly juvenile arthritis and systemic lupus erythematosus (11,12). The pathophysiologic mechanism of acquired HLH is not well understood; as genetic testing has improved, it has become evident that some cases may in fact represent fHLH (13). Interestingly, the same con-ditions associated with acquired HLH can trigger it in familial forms. Given the increasingly blurred distinction between familial and acquired forms of HLH, some believe that the syndrome should not be classified into distinct subtypes. Rather, there may be a continuum of risk to developing HLH, including both genetic and envi-ronmental factors (14). Regardless of etiology, HLH remains a syndrome diag-nosed by a unique pattern of clinical findings. HLH diagnostic guidelines have been published (15) (Table 1) and our patient exhibited 7 of the 8 diagnostic criteria: fever, splenomegaly, hypertriglyceridemia, hemophagocyto-sis, absent NK cell activity, elevated ferritin, and elevated soluble interleukin-2 receptor. Although not listed as part of the diagnostic criteria, neurological symptoms can predominate the initial clinical presentation of HLH. Deiva et al (16) reported that approx-imately 63% of children have neurologic symptoms at the onset of fHLH, including irritability, seizures, impaired consciousness, meningismus, and cranial nerve palsies. Parenchymal edema of the brain, which may occur in HLH, can affect the cerebellum (17,18). In the most extreme cases, this can lead to fatal tonsillar herniation. Neuropathologic staging of HLH has been established and correlated with clinical manifestations: Stage 1-leptomeningeal infiltrates of lymphocytes and histiocytes; Stage 2-adjacent parenchymal involvement with perivascular infiltration; Stage 3-massive parenchymal infiltration via lymphocytes and histiocytes, with tissue necrosis (19). These cellular changes lead to neuroimaging findings, including edema, focal areas of necrosis with parenchymal volume loss, diffuse white matter abnormalities, and leptomeningeal or perivascular enhancement (20). There is a single report of vertical nystagmus in a patient with HLH (21). This occurred in a 13-month-old boy accompanied by a bulging fontanelles, papilledema, and CSF pleocytosis. Computed tomography of the brain showed "no specific abnormality," and the patient's condi-tion improved with steroid therapy. Treatment of HLH to stabilize signs and symptoms involves the use of immunosuppressive agents, including dexamethasone, etoposide, cyclosporine A, and intrathecal methotrexate (15). Curative therapy is HSCT (22). Even FIG. 3. After treatment, noncontrast T1 sagittal magnetic resonance imaging shows resolution of both cerebellar swelling and downward displacement of the cerebellar ton-sils. TABLE 1. 2004 Diagnostic guidelines for HLH The diagnosis HLH can be established if one of either 1 or 2 below is fulfilled (1) A molecular diagnosis consistent with HLH (2) Diagnostic criteria for HLH fulfilled (5 of the 8 criteria below) Fever Splenomegaly Cytopenias (affecting $2 of 3 lineages in the peripheral blood): Hemoglobin ,90 g/L (in infants ,4 wk: hemoglobin ,100 g/L) Platelets ,100 · 109/L Neutrophils ,1.0 · 109/L Hypertriglyceridemia and/or hypofibrinogenemia: Fasting triglycerides $3.0 mmol/L (i.e., $265 mg/dL) Fibrinogen #1.5 g/L Hemophagocytosis in bone marrow or spleen or lymph nodes Low or absent NK cell activity Ferritin $500 mg/L Soluble CD25 (i.e., soluble IL-2 receptor) $2400 U/mL Adapted from Henter et al (15). Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation. HLH, hemophagocytic lymphohistiocytosis; IL, interleukin; NK cell, natural killer cell. Cai et al: J Neuro-Ophthalmol 2014; 34: 57-60 59 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. with HSCT, the estimated overall 3-year probability of survival has been reported at approximately 64% (15). Reduced-intensity conditioning HSCT has significantly improved survival, with the current reports of approxi-mately 92% survival at 3 years (23). Despite these recent advances, untreated HLH is still a rapidly fatal disease that warrants urgent diagnosis and intervention. Our case is unique in both documenting the occurrence of DBN in HLH and the resolution of DBN with treatment of cerebellar edema. REFERENCES 1. Farquhar J, Claireaux A. Familial haemophagocytic reticulosis. Arch Dis Child. 1952;27:519-525. 2. Henter JI, Elinder G, Söder O, Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80:428-435. 3. Niece JA, Rogers ZR, Ahmad N, Langevin AM, McClain KL. Hemophagocytic lymphohistiocytosis in Texas: observations on ethnicity and race. Pediatr Blood Cancer. 2010;54:424-428. 4. Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew PA, Henter JI, Bennett M, Fischer A, de Saint Basile G, Kumar V. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957-1959. 5. Freeman HR, Ramanan AV. Review of haemophagocytic lymphohistiocytosis. Arch Dis Child. 2011;96:688-693. 6. Filipovich AH. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol. 2011;11:512-516. 7. Pachlopnik Schmid J, Cote M, Menager M, Burgess A, Nehme N, Menasche G, Fischer A, de Saint Basile G. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235:10-23. 8. Gupta S, Weitzman S. Primary and secondary hemophagocytic lymphohistiocytosis: clinical features, pathogenesis and therapy. Expert Rev Clin Immunol. 2010;6:137-154. 9. Bhasin A, Tolan RW. Hemophagocytic lymphohistiocytosis- a diagnostic dilemma: two cases and review. Clin Pediatr (Phila). 2013;52:297-301. 10. Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245-253. 11. Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166:95-109. 12. Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22:561-566. 13. Nagafuji K, Nonami A, Kumano T, Kikushige Y, Yoshimoto G, Takenaka K, Shimoda K, Ogha S, Yasukawa M, Horiuchi H. Perforin gene mutations in adult-onset hemophagocytic lymphohistiocytosis. Haematologica. 2007;92:978-981. 14. Risma K, Jordan MB. Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr. 2012;24:9-15. 15. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. 16. Deiva K, Mahlaoui N, Beaudonnet F, De Saint Basile G, Caridade G, Moshous D, Mikaeloff Y, Blanche S, Fischer A, Tardieu M. CNS involvement at the onset of primary hemophagocytic lymphohistiocytosis. Neurology. 2012;78:1150-1156. 17. Astigarraga I, Prats JM, Navajas A, Fernández-Teijeiro A, Urberuaga A. Near fatal cerebellar swelling in familial hemophagocytic lymphohistiocytosis. Pediatr Neurol. 2004;30:361-364. 18. Chiapparini L, Uziel G, Vallinoto C, Bruzzone MG, Rovelli A, Tricomi G, Bizzi A, Nardocci N, Rizzari C, Savolardo M. Hemophagocytic lymphohistiocytosis with neurological presentation: MRI findings and a nearly miss diagnosis. Neurol Sci. 2011;32:473-477. 19. Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. 1997;130:358-365. 20. Goo HW, Weon YC. A spectrum of neuroradiological findings in children with haemophagocytic lymphohistiocytosis. Pediatr Radiol. 2007;37:1110-1117. 21. Henter JI, Elinder G. Cerebromeningeal haemophagocytic lymphohistiocytosis. Lancet. 1992;339:104-107. 22. Marsh RA, Jordan MO, Filipovich AH. Reduced-intensity conditioning haematopoietic cell transplantation for haemophagocytic lymphohistiocytosis: an important step forward. Br J Haematol. 2011;154:556-563. 23. Marsh RA, Vaughn G, Kim MO, Li D, Jodele S, Joshi S, Mehta PA, Davies SM, Jordan MB, Bleesing JJ, Filipovich AH. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824-5831. 60 Cai et al: J Neuro-Ophthalmol 2014; 34: 57-60 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |