| OCR Text |
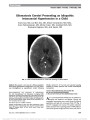
Show Subacute Bilateral Visual Loss in Methylmalonic Acidemia Ghislaine Traber, MD, Matthias R. Baumgartner, MD, Urs Schwarz, MD, Athina Pangalu, MD, Marc Y. Donath, MD, Klara Landau, MD Abstract: A 23-year-old woman known to have methylma-lonic acidemia (MMA) since birth suffered bilateral visual loss within 5 days. Multiple sclerosis, Leber hereditary optic neuropathy, vasculitis, infections (in particular treponema), and vitamin deficiency were ruled out. The optic nerve head changed from normal in appearance to atrophic. Treatment attempts with high-dose intravenous steroids and coenzyme Q10 combined with vitamin E were ineffective. The patient's underlying disease was metabolically well controlled by strict diet and carnitine supplementation. Toxic damage of both optic nerves due to MMA is the most likely mechanism. MRI showed moderate enhancement of both optic nerves. To our knowledge, this is the first report of a morphological correlate on MRI. Journal of Neuro-Ophthalmology 2011;31:344-346 doi: 10.1097/WNO.0b013e31822db480 © 2011 by North American Neuro-Ophthalmology Society A23-year-old woman known to have methylmalonic acidemia (MMA) since birth suffered bilateral visual loss over 5 days. Her disease was well controlled by strict diet and carnitine supplementation. Multiple sclerosis, Leb-er hereditary optic neuropathy (LHON), and infectious, nutritional, and vasculitic causes were ruled out. MRI showed enhancement of both optic nerves. Toxic damage of both optic nerves due to MMA was the most likely mechanism. Treatment with high-dose intravenous cortico-steroids and coenzyme Q10 combined with vitamin E was ineffective. Although optic nerve involvement previously has been reported in MMA, the neuroimaging findings in our case make it unique. MMA is a rare metabolic disease of autosomal recessive inheritance resulting in mitochondrial dysfunction. Affected patients commonly present in the neonatal period or in early infancy with a severe metabolic encephalopathy, metabolic acidosis, failure to thrive, developmental delay, various neurological symptoms, and eventually multiorgan dysfunction (1). The disorder is caused by deficient activity of the enzyme methylmalonyl-CoA-mutase or by defects of intracellular synthesis of its cofactor adenosylcobalamin (co-enzyme form of vitamin B12). The deficiencies caused by mutations in the apomutase locus are further subdivided into defects without activity (mut0) and defects with resid-ual activity (mut−). Impaired degradation of the amino acids (valine, isoleucine, methionine, and threonine), of odd-chain fatty acids and cholesterol side chains, and of thymine and uracil results in an accumulation of methylmalonic acid and other toxic metabolites. This is thought to cause sec-ondary mitochondrial dysfunction (2). Preclinical studies and tissue analyses from patients with MMA suggest that mitochondrial impairment occurs through a combination of inhibition of specific enzymes and transporters, limitation in the availability of substrates for mitochondrial pathways, and oxidative damage (3). Dis-ruption of mitochondrial homeostasis may lead to impair-ment of energy metabolism and further increase in reactive oxygen species due to reduced electron flow in the mito-chondrial respiratory chain (4). Therapy is mainly based on a diet low in propionic amino acids and high in energy (1). There have been reports of optic atrophy associated with organic acidurias in early childhood (5,6). Late-onset optic neuropathies in adolescence or adulthood associated with MMA have been reported by 2 groups with ages ranging from 15 to 21 years (7,8). In our patient, optic neuropathy developed later but more rapidly and showed a morpholog-ical correlate on MRI. Departments of Ophthalmology (GT, KL), Neurology (US), Neuro-radiology (AP), and Endocrinology, Diabetes and Clinical Nutrition (MYD), University Hospital Zurich, Zurich, Switzerland; and Department of Metabolism (MRB) and Children's Research Center (MRB), University Children's Hospital Zurich, Zurich, Switzerland. The authors report no conflicts of interest. Address correspondence to Klara Landau, MD, Department of Ophthalmology, University Hospital of Zurich, Frauenklinikstrasse 24, CH-8091 Zurich, Switzerland; E-mail: Klara.Landau@usz.ch 344 Traber et al: J Neuro-Ophthalmol 2011; 31: 344-346 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. CASE REPORT Our patient is a 23-year-old woman with MMA who 4 days after birth developed generalized hypotonia, hypothermia, and dyspnea. Diagnosis of MMA was confirmed by severely decreased methylmalonyl-CoA-mutase activity in cultu-red fibroblasts and later by compound heterozygosity for p.A137V/p.N219Y in the MUT gene, compatible with a mut0 defect (Patient 006 in Lempp et al, 2007 [9]). She had a mild developmental delay, impaired renal func-tion, a hypothalamo-hypophyseal insufficiency with growth retardation, and a history of abnormal puberty. The patient experienced bilateral visual loss with rapid deterioration over 5 days. Initial neuro-ophthalmic exam-ination 5 days after the onset of symptoms revealed finger counting vision in each eye with large pupils that reacted poorly to light; slight, bilateral, posterior subcapsular cataracts; cecocentral scotomas on kinetic perimetry, and normal fundi. The neurological examination was otherwise normal. An electroencephalogram was normal, and visual evoked potentials showed prolonged latency of the P100 component bilaterally. MRI performed 2 weeks later showed enhancement of both optic nerves (Fig. 1) and symmetric T2-hyperintense lesions in the posterior limb of each internal capsule. Given the possibility of an inflammatory optic neurop-athy, high-dose intravenous steroid therapy was adminis-tered for 5 days without improvement. Testing for LHON, vasculitis, infections, including syphilis, and vitamin B1, B6, B12, and folic acid deficiency was all normal. Cerebrospinal fluid analysis was unremarkable. MRI of the spine and neuromyelitis optica antibody testing were not performed. Supplementation with coenzyme Q10 (180 mg/d) combined with vitamin E (200 mg/d) started 7 months after onset of symptoms failed to improve visual function. Three months after the onset of vision loss, the patient developed subacute partial neurosensory hearing loss. At 6 months, she experienced a further decline in vision to hand motions in each eye and the optic discs were found to be diffusely pale. At 9 months, vision remained unchanged and the patient remained metabolically well controlled, adhering to a therapeutic diet and carnitine supplementation. DISCUSSION Ophthalmologic findings in MMA infrequently have been reported. Cataract formation has been described by Stromme et al (10) in 2 siblings with atypical MMA. De Baulny et al (5) mentioned 2 patients who developed optic atrophy and neurosensorial deafness but did not provide clinical details. Severe optic neuropathy in MMA has been reported in 3 patients. Williams et al (8) described 2 men with vision loss over 3-4 weeks: a 16-year-old with acuity of 20/300, right eye, and 20/150, left eye, and a 21-year-old with acuity of 20/200 in each eye. Pinar-Sueiro et al (7) reported a 15-year-old girl with vision loss to 20/400, right eye, and 20/40, left eye, occurring over 5 weeks. These patients underwent either CT or MRI without detection of optic nerve abnormalities. We postulate that the underlying cause for bilateral optic neuropathy and neurosensorial hearing loss in our patient is due to a delayed progressive breakdown of mitochondrial function with induction of neuronal cell death. This hypothesis (8) is supported by growing evidence of in vitro and in vivo studies suggesting an inhibition of mitochondrial metabolism in MMA (2,3), as well as the similarity to the clinical profile of LHON and other mito-chondrial optic neuropathies (11). The reason for the delayed onset of optic neuropathy in patients with MMA and the closely related condition of propionic acidemia (PA) may be due to the fact that only low amounts of methylmalonic acid (only in MMA) and other dicarboxylic acids (in MMA and PA) are produced in the brain. Since the blood-brain barrier is virtually imper-meable to these dicarboxylic acids, they slowly accumulate within the central nervous system and induce delayed mi-tochondrial dysfunction (2,3). The resulting ATP depletion with collapse of ion gradients may result in Ca++ influx and induction of mitochondrial permeability transition (MPT), FIG. 1. Contrast-enhanced T1 axial (A) and coronal (B) MRI reveals enhancement (arrows) of both optic nerves. Traber et al: J Neuro-Ophthalmol 2011; 31: 344-346 345 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. which induces apoptosis (12). Oxidative stress is an impor-tant inducer of MPT as well (3,13). With loss of cellular homeostasis and cell death, there is a breakdown of the blood-brain barrier. This may explain the optic nerve en-hancement seen on MRI in our patient. Currently, there is no effective therapy for MMA. Treatment with coenzyme Q10 and vitamin E have been described (7,8). Coenzyme Q10, an essential cofactor in the mitochondrial respiratory chain, has gained attention for its neuroprotective properties and its potential role in the treat-ment of neurodegenerative (14) and mitochondrial (15) dis-eases. Its neuroprotective effect is related to antioxidant activity and to a specific regulation of the MPT pore (16,17). Coenzyme Q10 has been shown to be reduced in fibroblasts of patients with MMA (18). Vitamin E is a well-known free radical scavenger (19,20). While Williams et al (8) were unable to show any benefit with the use of coen-zyme Q10, Pinar-Sueiro et al (7) reported a significant im-provement in visual function after treatment with coenzyme Q10 (200 mg/d) and vitamin E (200 mg/d). However, this treatment regimen was ineffective in our patient. REFERENCES 1. Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C:104-112. 2. Morath MA, Okun JG, Muller IB, Sauer SW, Horster F, Hoffmann GF, Kolker S. Neurodegeneration and chronic renal failure in methylmalonic aciduria-a pathophysiological approach. J Inherit Metab Dis. 2008;31:35-43. 3. Melo DR, Kowaltowski AJ, Wajner M, Castilho RF. Mitochondrial energy metabolism in neurodegeneration associated with methylmalonic acidemia. J Bioenerg Biomembr. 2011;43:39-46. 4. Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333-343. 5. de Baulny HO, Benoist JF, Rigal O, Touati G, Rabier D, Saudubray JM. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28:415-423. 6. Ianchulev T, Kolin T, Moseley K, Sadun A. Optic nerve atrophy in propionic acidemia. Ophthalmology. 2003;110:1850-1854. 7. Pinar-Sueiro S, Martinez-Fernandez R, Lage-Medina S, Aldamiz- Echevarria L, Vecino E. Optic neuropathy in methylmalonic acidemia: the role of neuroprotection. J Inherit Metab Dis. 2010; May 7 (epub ahead of print). doi: 10.1007/s10545- 010-9084-8. 8. Williams ZR, Hurley PE, Altiparmak UE, Feldon SE, Arnold GL, Eggenberger E, Mejico LJ. Late onset optic neuropathy in methylmalonic and propionic acidemia. Am J Ophthalmol. 2009;147:929-933. 9. Lempp TJ, Suormala T, Siegenthaler R, Baumgartner ER, Fowler B, Steinmann B, Baumgartner MR. Mutation and biochemical analysis of 19 probands with mut0 and 13 with mut− methylmalonic aciduria: identification of seven novel mutations. Mol Genet Metab. 2007;90:284-290. 10. Stromme P, Stokke O, Jellum E, Skjeldal OH, Baumgartner R. Atypical methylmalonic aciduria with progressive encephalopathy, microcephaly and cataract in two siblings- a new recessive syndrome? Clin Genet. 1995;48:1-5. 11. Sadun AA. Mitochondrial optic neuropathies. J Neurol Neurosurg Psychiatry. 2002;72:423-425. 12. Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649-H657. 13. Castilho RF, Kowaltowski AJ, Meinicke AR, Bechara EJ, Vercesi AE. Permeabilization of the inner mitochondrial membrane by Ca2+ ions is stimulated by t-butyl hydroperoxide and mediated by reactive oxygen species generated by mitochondria. Free Radic Biol Med. 1995;18:479-486. 14. Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr Dis Treat. 2009;5:597-610. 15. DiMauro S, Hirano M, Schon EA. Approaches to the treatment of mitochondrial diseases. Muscle Nerve. 2006;34:265-283. 16. Russo R, Cavaliere F, Rombola L, Gliozzi M, Cerulli A, Nucci C, Fazzi E, Bagetta G, Corasaniti MT, Morrone LA. Rational basis for the development of coenzyme Q10 as a neurotherapeutic agent for retinal protection. Prog Brain Res. 2008;173:575-582. 17. Nucci C, Tartaglione R, Cerulli A, Mancino R, Spano A, Cavaliere F, Rombola L, Bagetta G, Corasaniti MT, Morrone LA. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397-406. 18. Haas D, Niklowitz P, Horster F, Baumgartner ER, Prasad C, Rodenburg RJ, Hoffmann GF, Menke T, Okun JG. Coenzyme Q (10) is decreased in fibroblasts of patients with methylmalonic aciduria but not in mevalonic aciduria. J Inherit Metab Dis. 2009;32:570-575. 19. Sokol RJ, McKim JMJ, Goff MC, Ruyle SZ, Devereaux MW, Han D, Packer L, Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164-174. 20. Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709-716. 346 Traber et al: J Neuro-Ophthalmol 2011; 31: 344-346 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |