| OCR Text |
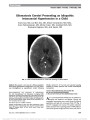
Show Nystagmus and Ataxia Associated With Antiganglioside Antibodies Seong-Hae Jeong, MD, Jungmoo Nam, MD, Min Jeong Kwon, PhD, Jong Kuk Kim, MD, PhD, Ji Soo Kim, MD, PhD Background: Antiganglioside antibodies are found in vari-ous neurological disorders that constitute a continuum from peripheral neuropathy to encephalitis. However, nystagmus has rarely been described in patients with ataxia associated with antiganglioside antibodies. Methods: From January 2008 to July 2009, we identified 3 patients with acute ataxia and nystagmus in 2 University Hospitals of Korea, who were found to have anti-GD1b, anti- GM1, or anti-GQ1b antibodies. Results: In addition to acute ataxia, all 3 patients showed various combinations of nystagmus, which included central positional nystagmus (n = 3), vertical nystagmus (n = 1), and periodic alternating nystagmus (n = 1). The spontane-ous and positional nystagmus were mostly detectable only with the elimination of fixation and magnification of the eyes using video goggles. Two patients also exhibited gaze-evoked nystagmus that was noticeable without the aid of video goggles. Patients had serum IgG antibodies to GD1b, GM1, or GQ1b. Cerebrospinal fluid examination, nerve con-duction studies, and brain MRI were normal. In all patients, the symptoms and signs resolved over 3-12 months. Conclusions: Various forms of nystagmus with acute ataxia may be a sole or predominant manifestation of disorders related to antiganglioside antibodies. The nystagmus indi-cates a central pathology involving the cerebellum or brainstem in this antibody-associated disorder. Antiganglio-side antibodies should be measured in patients with nystagmus and acute ataxia of undetermined etiology. Journal of Neuro-Ophthalmology 2011;31:326-330 doi: 10.1097/WNO.0b013e31822f6707 © 2011 by North American Neuro-Ophthalmology Society Gangliosides, sialic acids containing glycosphingolipids, are diverse and highly complex molecules located pri-marily on the plasma membranes of the nervous system (1,2). Gangliosides play important roles in biological func-tions, such as cellular growth and differentiation, modula-tion of signal transduction, and immune reactions (3). Antibodies to gangliosides have been found in the neurop-athy associated with IgM paraproteinemia (4), multifocal motor neuropathy (5), chronic inflammatory demyelinating polyneuropathy (6), Fisher syndrome, Guillian-Barré syn-drome (GBS) (7), and Bickerstaff brainstem encephalitis (8). In particular, GQ1b, GD1b, and GM1 are the antigens frequently recognized by such serum antibodies. Several studies have also reported dense distribution of GQ1b at the dorsal root ganglion and paranodal myelin of the cranial nerves innervating extraocular muscles (9). These findings may explain the frequent observation of ophthalmoplegia and ataxia in patients with anti-GQ1b antibody. Although the distribution of GM1 and GD1b is largely unknown in human central nervous system (10,11), serum antibodies against GM1 or GD1b are frequently detected in autoim-mune neuropathies, such as multifocal motor neuropathy, IgM paraproteinemic neuropathy, and GBS. Furthermore, IgG anti-GD1b antibody is closely associated with sensory or cerebellar-type ataxia in patients with GBS (12,13). Ataxia and ophthalmoplegia have been associated with anti-GQ1b antibody in Fisher syndrome, GBS with ophthalmoplegia, and Bickerstaff brainstem encephalitis (14,15). Also, ophthalmoplegia has been reported in asso-ciation with anti-GM1 and anti-GD1b IgM antibodies (16,17). However, ocular oscillations have been described only in 2 patients with antiganglioside antibodies. One Department of Neurology (S-HJ), Chungnam National University Hospital, Daejeon, South Korea; Department of Neurology (JN, JSK), Seoul National University College of Medicine, Seoul National Uni-versity Bundang Hospital, Seongnam, South Korea; Department of Radiology (MJK), Daejeon St Mary's Hospital, The Catholic Univer-sity of Korea, Daejeon, South Korea; and Department of Neurology (JKK), School of Medicine, Dong-A University, Busan, South Korea. Supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A080750). Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com) The authors report no conflicts of interest. Address correspondence to Ji Soo Kim, MD, PhD, Department of Neurology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, 300 Gumi-dong, Bundang-gu, Seongnam-si, Gyeonggi-do 463-707, South Korea; E-mail: jisookim@snu.ac.kr 326 Jeong et al: J Neuro-Ophthalmol 2011; 31: 326-330 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. patient with sensory dominant polyneuropathy and cerebel-lar ataxia developed downbeat nystagmus in the presence of serum IgM protein that specifically bound to GM1, GD1b, and asialo-GM1 (18). Ocular flutter was reported in another patient with anti-GQ1b antibody-associated ataxia and myoclonus (19). We describe nystagmus in 3 patients with autoantibodies against gangliosides GD1b, GM1, and GQ1b. METHODS Subjects We identified 3 patients with dizziness, nystagmus, and ataxia in association with antiganglioside antibodies in 2 university hospitals in Korea from January 2008 to July 2009 (Table 1). This study received approval from the Institutional Review Board of the Seoul National University Bundang Hospital. Neurological Evaluation Patients received a bedside neurological evaluation, in-cluding spontaneous, gaze-evoked (GEN), head-shaking, and positional/positioning nystagmus, in addition to rou-tine neurological testing. Spontaneous and positional/ positioning nystagmus were observed with fixation and after eliminating fixation and magnifying the eyes using binocular video goggles (SLMED, Seoul, South Korea). GEN was induced by fixating on an eccentric target in the horizontal and vertical directions without using video goggles. For positional/positioning nystagmus, patients were asked to look at their knees with their head bent down, straightened, and turned to either side while sitting. Patients were also subjected to lying down, turning their head to either side while supine, straight head hanging, and Dix- Hallpike maneuvers (20). In 2 patients, eye movements were also recorded using 3-dimensional video-oculography (SMI, Teltow, Germany). Measurements of Antiganglioside Antibodies Serum samples were obtained from the patients during the acute phase. The samples were analyzed for the presence of IgG antibodies against GQ1b, GD1b, and GM1. Detailed methods on measurement of anti-GQ1b antibody have been described previously (21). The presence of anti-GD1b and anti-GM1 antibodies was determined using an enzyme-linked immunosorbent assay kit (IMMCO Diagnostics, Inc, Buffalo, NY) according to the manufacturer's instructions. RESULTS Clinical Course Our patients suffered from dizziness/vertigo and imbalance that developed either suddenly or over a few days and improved over several months. Patients were young (age range = 16-32 years) and in excellent health. Only 1 patient reported a preceding upper respiratory infection before the development of dizziness and imbalance (Table 1). In all patients, the symptoms and signs resolved over 3 to 12 months without specific treatment. Patterns of Nystagmus Along with GEN, patients showed central positional nystag-mus (CPN), upbeat and downbeat nystagmus, or periodic alternating nystagmus (PAN) (Table 1). With the exception of CPN in Case 1, the spontaneous and positional nystagmus were noticeable only with the elimination of fixation and magnification of the eyes using video goggles. GEN was present only during horizontal gaze in one patient (Case 2), whereas it was induced during both horizontal and verti-cal gazes in another patient (Case 1). CPN took various forms, but 2 patients showed direction-changing positional nystagmus, apogeotropic in one (Case 1) and geotropic in the other (Case 3). In Case 2, the spontaneous upbeat nystagmus changed to downbeat with head bending. Case 2 also showed evolution of nystagmus from PAN and GEN to CPN and then to upbeat nystagmus. Case 3 also developed positional upbeat nystagmus during follow-up. Antiganglioside Antibodies Patients showed serum IgG antibodies to GD1b, GM1, or GQ1b (Table 1). One patient (Case 2) also showed findings TABLE 1. Clinical profile of patients with antiganglioside antibodies Patient/ Age/ Sex Preceding Illness Nystagmus Laboratory Findings Anti- GD1b Anti-GMI Anti- GQ1b CSF (cells/mm3)/ protein (mg/dL) NCS 1/32/F URI H/V-GEN, CPN Positive Negative Negative 0/49.7 Normal 2/16/M - PAN, downbeat, upbeat, GEN, CPN Negative Negative Positive 0/37 Normal 3/29/M - CPN Positive Positive Negative ND ND Downbeat, downbeat nystagmus; F, female; H/V, horizontal/vertical; M, male; ND, not done; upbeat, upbeat nystagmus; URI, upper respiratory infection. Jeong et al: J Neuro-Ophthalmol 2011; 31: 326-330 327 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. suggestive of recent Epstein-Barr virus (EBV) infection (see Report of Cases). Other Findings Other than ataxia and nystagmus, patients showed normal neurological examination, except mild bifacial and right arm weakness, and dysarthria in 1 patient (Case 1). All patients had full ocular motility, intact sensory examination, and normal deep tendon reflexes. Cerebrospinal fluid (CSF) examination was performed in 2 patients, and the results were normal. Nerve conduction study (NCS) was also normal in 2 patients. Brain MRI in all patients did not show any abnormality, which would explain their symptoms and signs. Report of Cases Case 1 A 32-year-old woman was referred for evaluation of vertigo and imbalance for 2 months. She was treated with a canalith repositioning maneuver for presumed benign paroxysmal positional vertigo (BPPV). Over the following month, her balance worsened and she was unable to sit unaided. The patient then gradually improved and was able to walk with a cane. Examination showed GEN, which beat leftward, down-ward, and counterclockwise during the leftward gaze and beat rightward during the rightward gaze. Vertical gaze evoked nystagmus in the direction of gaze. Horizontal smooth pursuit was impaired bilaterally, and saccades were hypometric. Head-bending while sitting induced left-beating nystagmus with a latency of 5 seconds, which, on lying down, converted to prominent right-beating nystagmus with associated vertigo lasting up to 8 minutes. Quick head rotation to either side through 90° while supine induced direction-changing apogeo-tropic nystagmus (Supplemental video 1, Supplemental Dig-ital Content 1; http://links.lww.com/WNO/A21). (Moving from sitting to supine position immediately induces promi-nent right-beating nystagmus that lasts for more than 5 minutes. Quick head rotation to either side through 90° while supine evokes direction-changing apogeotropic nys-tagmus, more marked during left head turning. Head-bend-ing while sitting generates left-beating nystagmus with a latency of 5 seconds. The nystagmus was recorded with the elimination of fixation but could be observed even with fixation.) The positional nystagmus could be observed with fixation, but increased with the elimination of fixation. On neurological testing, the patient had mild bilateral facial di-plegia, dysarthria, right arm weakness, head titubation, dys-metria, dysdiadochokinesia, and ataxic gait. CSF examination and brain MRI were normal. Anti- GD1 IgG antibody was increased (37.5 EU/mL, normal range: ,20 EU/mL) while the titers for anti-GQ1b and anti-GM1 antibodies were within normal range. NCS per-formed 70 days in her clinical course was normal. The patient improved without specific treatment and showed only subtle dysmetria without vertigo or positional nystag-mus 4 months after the onset of symptoms. Case 2 A 16-year-old student presented with vertigo and imbal-ance for 2 weeks. Initially, he showed downbeat nystagmus and PAN along with horizontal GEN. The PAN reversed its direction every few seconds with a transition period of 1-2 seconds (Fig. 1; Supplemental video 2, Supplemental Digital Content 2; http://links.lww.com/WNO/A22). (The video shows horizontal-downbeat nystagmus. The horizontal nystagmus reverses its direction every few seconds with a minimal transition period [PAN] while downbeat nys-tagmus persists. The nystagmus was detectable only after FIG. 1. Case 2. A. Without fixation, video-oculographic re-cording of PAN shows a mixed horizontal-downbeat nystag-mus, with the horizontal component reversing its direction every few seconds with a transition period of 1-2 seconds. Upward deflection indicates rightward or upward eye motion. B. Temporal profile of horizontal SPV of the PAN discloses a crescendo-decrescendo pattern with an approximate os-cillation period of 8 seconds. Positive values represent rightward eye motion. LH, horizontal position of the left eye; LV, vertical position of the left eye; RH, horizontal position of the right eye; RV, vertical position of the right eye. 328 Jeong et al: J Neuro-Ophthalmol 2011; 31: 326-330 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. eliminating fixation and magnifying the eyes using binocular video goggles.) The downbeat nystagmus and PAN were de-tectable only after eliminating fixation and magnifying the eyes using video goggles. Saccades were hypometric, and smooth pursuit was impaired in both horizontal and vertical directions. The patient had gait ataxia without limb dysme-tria. IgG antibody to GQ1b was elevated in the serum at 73.5% (normal range: ,20%). Although he showed positive IgM antibody titer in serum for viral capsid antigen of Epstein-Barr virus (EBV-VCA), polymerase chain reaction for EBV was negative. CSF examination, NCS, and brain MRI were normal. One week later, the patient had positional nystagmus detected with video goggles only with removal of fixation. The nystagmus was geotropic during head turning to either side while supine and during Dix-Hallpike maneuvers. Two months later, PAN and GEN disappeared with im-provement of vertigo and imbalance. Subtle right-beating nystagmus developed with the elimination of fixation using left head turn in the supine position, and subtle upbeat nystagmus occurred during left Dix-Hallpike maneuver. Seven months after the symptom onset, the patient reported only mild dizziness with video goggles and showed sponta-neous upbeat nystagmus with a slow-phase velocity (SPV) of 6.0°/s, which converted into downbeat nystagmus (SPV at 6.2°/s) with head bending. One year later, the neuro-ophthalmic examination was normal, and the patient de-nied dizziness or imbalance. Case 3 A 29-year-old man complained of dizziness and imbalance for 2 months. Initially, he was diagnosed with BPPV involving the right horizontal canal and received a canalith repositioning maneuver. At that time, he had right-beating nystagmus on rightward head turning while supine. One month later, the patient no longer had positional nystagmus. Two months after developing symptoms, examination with video goggles demonstrated upbeat nystagmus induced by lying down and Dix-Hallpike maneuvers when the fix-ation was eliminated. The patient showed impaired smooth pursuit and leftward falling on enhanced Romberg test. Elevation levels of anti-GD1b (84.4 EU/mL, normal range: ,20 EU/mL) and anti-GM1 (53.8 EU/mL, normal range: ,20 EU/mL) IgG antibodies were found in the serum. One month later, patient reported resolution of dizziness and imbalance, and his examination showed no evidence of spontaneous or positional nystagmus. DISCUSSION Our patients presented with vertigo, ataxia, and various forms of nystagmus, including vertical, PAN, GEN, and positional nystagmus. The most striking immunological finding was the presence of serum antibodies to GD1b, GM1, or GQ1b. Anti-GD1b antibodies are commonly associated with the ataxic form of GBS (18,22,23). Our patients showed anti-GD1b or anti-GM1 antibodies without features of this disorder. The association of ataxia and nystagmus with these antibodies is supported by the finding that GD1b is present in the cerebellar granular area, dentate and olivary nuclei, sensory ganglia, and spinocerebellar Ia fibers of the periph-eral nerves (1,18). A previous study demonstrated that IgM M-protein from a patient with motor neuron disease had antibody activity against the gangliosides GM1, GD1b, and asialo-GM1 (24). In that report, the cerebellar granular cells and white matter were stained with patient's monoclonal IgM using immunohistochemical methods. These findings support the specific localization of gangliosides GM1 and GD1b both in the granular layer and in the white matter of the cerebellum (24,25). In our patients, involvement of the cerebellum is likely given the clinical findings of ataxia and various forms of nystagmus, including vertical nystagmus, PAN, GEN, and CPN (26,27). Acute or subacute cerebellar ataxia has been reported in patients with antiganglioside antibodies (13,14,19,22,23). It is unknown if the antiganglioside anti-bodies are pathogenic, protective, or reactive bystanders. In general, ataxia associated with antiganglioside anti-body has a favorable prognosis. Acquired PAN has been reported in association with a number of conditions (26-28), many of which involve the cerebellum. We could not find previous reports of an association between PAN and antiganglioside antibody. GEN is indicative of inade-quate neural integrator function and may be observed in disorders of the brainstem and cerebellum. This includes acute inflammatory autoimmune disorder of the central nervous system, such as Fisher syndrome (21). CPN is usually due to lesions in the caudal brainstem or vestibulo-cerebellum (27,29). CPN may have various patterns, including direction-changing nystagmus. Two of our patients (Cases 1 and 3) initially were diagnosed with BPPV involving the horizontal semicircular canal. This also may generate direction-changing nystagmus depending on head position (30). However, the associated cerebellar signs and failure to improve with repeated canalith repositioning maneuvers helped establish the central localization (31). One patient (Case 2) had elevated IgM antibody for EBV-VCA, indicating a recent infection (32). The clinical spectrum of EBV infection includes meningitis, meningo-encephalitis, and various neuromuscular complications (33). Possibly, this viral infection in our patient triggered an immune response generating antiganglioside antibodies. In our patients, the nystagmus was primarily detected when fixation was eliminated using video goggles. This may explain why nystagmus has rarely been reported in associa-tion with antiganglioside antibodies (18). For complete eval-uation of nystagmus, patients should be examined in various head and eye positions both with and without fixation and with binocular (magnifying) goggles, if available. Jeong et al: J Neuro-Ophthalmol 2011; 31: 326-330 329 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. REFERENCES 1. Kotani M, Kawashima I, Ozawa H, Terashima T, Tai T. Differential distribution of major gangliosides in rat central nervous system detected by specific monoclonal antibodies. Glycobiology. 1993;3:137-146. 2. Gong Y, Tagawa Y, Lunn MP, Laroy W, Heffer-Lauc M, Li CY, Griffin JW, Schnaar RL, Sheikh KA. Localization of major gangliosides in the PNS: implications for immune neuropathies. Brain. 2002;125:2491-2506. 3. Lopez PH, Schnaar RL. Gangliosides in cell recognition and membrane protein regulation. Curr Opin Struct Biol. 2009;19:549-557. 4. Freddo L, Ariga T, Saito M, Macala LC, Yu RK, Latov N. The neuropathy of plasma cell dyscrasia: binding of IgM M-proteins to peripheral nerve glycolipids. Neurology. 1985;35: 1420-1424. 5. Pestronk A, Cornblath DR, Ilyas AA, Baba H, Quarles RH, Griffin JW, Alderson K, Adams RN. A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Ann Neurol. 1988;24:73-78. 6. Sadiq SA, Thomas FP, Kilidireas K, Protopsaltis S, Hays AP, Lee KW, Romas SN, Kumar N, van den Berg L, Santoro M. The spectrum of neurologic disease associated with anti-GM1 antibodies. Neurology. 1990;40:1067-1072. 7. Yuki N. Fisher syndrome and Bickerstaff brainstem encephalitis (Fisher-Bickerstaff syndrome). J Neuroimmunol. 2009;30:1-9. 8. Ito M, Kuwabara S, Odaka M, Misawa S, Koga M, Hirata K, Yuki N. Bickerstaff's brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. 2008;255:674-682. 9. Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology. 1993;43:1911-1917. 10. Visser LH, Van der Meche FG, Van Doorn PA, Meulstee J, Jacobs BC, Oomes PG, Kleyweg RP, Meulstee J. Guillain-Barré syndrome without sensory loss (acute motor neuropathy). A subgroup with specific clinical, electrodiagnostic and laboratory features. Dutch Guillain-Barré Study Group. Brain. 1995;118(pt 4):841-847. 11. Jacobs BC, van Doorn PA, Schmitz PI, Tio-Gillen AP, Herbrink P, Visser LH, Hooijkass H, van der Meche FG. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann Neurol. 1996;40:181-187. 12. Pan CL, Yuki N, Koga M, Chiang MC, Hsieh ST. Acute sensory ataxic neuropathy associated with monospecific anti-GD1b IgG antibody. Neurology. 2001;57:1316-1318. 13. Sugimoto H, Wakata N, Kishi M, Fujioka T, Kurihara T, Irie Y, Saito T. A case of Guillain-Barré syndrome associated with cerebellar ataxia and positive serum anti-GD1b IgG antibody. J Neurol. 2002;249:346-347. 14. Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001;70:50-55. 15. Lee SH, Lim GH, Kim JS, Oh SY, Kim JK, Cha JK, Yun CH, Kang JK, Lee H, Song HK, Chung KC. Acute ophthalmoplegia (without ataxia) associated with anti-GQ1b antibody. Neurology. 2008;71:426-429. 16. Kwon HM, Hong YH, Sung JJ, Paeng JC, Lee DS, Lee KW. A case of Bickerstaff's brainstem encephalitis; the evidence of cerebellum involvement by SPM analysis using PET. Clin Neurol Neurosurg. 2006;108:418-420. 17. Arbogast SD, Khanna S, Koontz DW, Tomsak RL, Katirji B, Leigh RJ. Chronic ataxic neuropathy mimicking dorsal midbrain syndrome. J Neurol Neurosurg Psychiatry. 2007;78:1276-1277. 18. Hitoshi S, Kusunoki S, Chiba A, Takatsu R, Sunada Y, Nukina N, Tai T, Kanazawa I. Cerebellar ataxia and polyneuropathy in a patient with IgM M-protein specific to the Gal(beta 1-3)GalNAc epitope. J Neurol Sci. 1994;126:219-224. 19. Zaro-Weber O, Galldiks N, Dohmen C, Fink GR, Nowak DA. Ocular flutter, generalized myoclonus, and trunk ataxia associated with anti-GQ1b antibodies. Arch Neurol. 2008;65:659-661. 20. Jeong SH, Choi SH, Kim JY, Koo JW, Kim HJ, Kim JS. Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology. 2009;72:1069-1076. 21. Kim YK, Kim JS, Jeong SH, Park KS, Kim SE, Park SH. Cerebral glucose metabolism in Fisher syndrome. J Neurol Neurosurg Psychiatry. 2009;80:512-517. 22. Kaida K, Kamakura K, Ogawa G, Veda M, Motoyoshi K, Arita M, Kusunoki S. GD1b-specific antibody induces ataxia in Guillain- Barré syndrome. Neurology. 2008;71:196-201. 23. Yuki N, Susuki K, Hirata K. Ataxic form of Guillain-Barré syndrome associated with anti-GD1b IgG antibody. J Neurol Neurosurg Psychiatry. 2000;69:136-137. 24. Nardelli E, Steck AJ, Barkas T, Schluep M, Jerusalem F. Motor neuron syndrome and monoclonal IgM with antibody activity against gangliosides GM1 and GD1b. Ann Neurol. 1988;23:524-528. 25. Laev H, Rapport MM, Mahadik SP, Silverman AJ. Immunohistological localization of ganglioside in rat cerebellum. Brain Res. 1978;157:136-141. 26. Jeong HS, Oh JY, Kim JS, Kim J, Lee AY, Oh SY. Periodic alternating nystagmus in isolated nodular infarction. Neurology. 2007;68:956-957. 27. Leigh RJ, Zee DS. The Neurology of Eye Movements. 4th edition. New York, NY: Oxford University Press, 2006. 28. Jeong SH, Hwang JM, Kim JS. Co-occurrence of periodic alternating and pendular seesaw nystagmus in blindness. J Neurol Sci. 2009;285:257-258. 29. Watson P, Barber HO, Deck J, Terbrugge K. Positional vertigo and nystagmus of central origin. Can J Neurol Sci. 1981;8: 133-137. 30. Buttner U, Helmchen C, Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119:1-5. 31. Nam J, Kim S, Huh Y, Kim JS. Ageotropic central positional nystagmus in nodular infarction. Neurology. 2009;73:1163. 32. Evans AS, Niederman JC, McCollum RW. Seroepidemiologic studies of infectious mononucleosis with EB virus. N Engl J Med. 1968;279:1121-1127. 33. Portegies P, Corssmit N. Epstein-Barr virus and the nervous system. Curr Opin Neurol. 2000;13:301-304. 330 Jeong et al: J Neuro-Ophthalmol 2011; 31: 326-330 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |