| OCR Text |
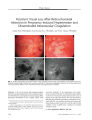
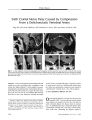
Show ORIGINAL CONTRIBUTION Anti- Hu Paraneoplastic Syndrome Presenting as Bilateral Sixth Cranial Nerve Palsies Torek Hammam, FRCS, Robert M. McFadzean, MD, and James W. Ironside Abstract: A 62- year- old woman presented with diplopia caused by bilateral sixth cranial nerve palsies. Two weeks later, she had bulbar weakness and ataxia. Brain magnetic resonance imaging showed non- specific abnormalities and spinal fluid was acellular but contained an elevated protein and oligoclonal bands. A paraneoplastic screen showed anti- Hu antibodies. Her clinical condition improved with immunoglobulin and systemic corticosteroid treatment. Breast cancer was diagnosed 21 months later by mammography but there were no metastases detected. Four and half years after the onset of her diplopia, she died of diffuse metastatic breast cancer. This is the first reported case of anti- Hu paraneoplastic brain stem encephalitis presenting with sixth cranial nerve palsies. ( J Neuro- Ophthalmol 2005; 25: 101- 104) The paraneoplastic syndromes are a group of rare immune- mediated disorders associated with cancer ( 1). High- titer autoantibodies may be found in the patient's serum and cerebrospinal fluid that are directed against neurons and the primary tumor. These autoantibodies are considered to be the result of an immunologic response to the primary tumor and may cross- react with cells of the nervous system, causing neuronal damage. Specific forms of this syndrome are associated with specific anti- neuronal antibodies and tumors. Because the onset of neurologic symptoms often precedes the cancer diagnosis, detection of these antibodies greatly assists investigation for the underlying tumor ( 1). Paraneoplastic encephalomyelitis/ sensory neuropathy presents with a wide spectrum of neurologic symptoms and signs associated with the anti- Hu antibody ANNA 1 ( anti- neuronal nuclear antibody type 1) in 53% of patients. The term " anti- Hu syndrome" is used for patients with the anti- Hu antibody ( 2). Other antibodies linked with this Department of Neuro- Ophthalmology, Institute of Neuroscience, Southern General Hospital, Glasgow, Scotland. Address correspondence to Tarek Hammam, FRCS, 22 Kirkmichael avenue, Hyndland, Glasgow Gil 7QQ, Scotland; E- mail: Thammam@ aol. com syndrome include ANNA3, anti- CV2/ CRMP5 ( collapsing response mediator protein- 5), anti- Ta/ Ma2, and anti-amphiphysin ( 3). The clinical spectrum includes diffuse encephalomyelitis, limbic and brain stem encephalitis, cer-ebellitis, and sensory, motor, and autonomic neuropathies ( 4). The most frequent underlying tumor is a small cell lung cancer ( SCLC). Non- SCLC, neuroblastoma, and cancer of the prostate, stomach, breast, and adrenal gland have been also reported in this syndrome ( 1). On immunohistochemical screening for the anti- Hu antibody, the characteristic features are strong staining of the nucleus sparing the nucleolus and faint staining of the cytoplasm in neurons of the central and peripheral nervous system ( 1). The anti- Hu antibody reacts not only with the patient's own tumor but also with the small cell lung cancer of other patients. We report a patient with anti- Hu paraneoplastic brain stem encephalitis who presented with diplopia caused by bilateral sixth nerve palsies. CASE REPORT A 62- year- old woman presented with double vision, followed 2 weeks later by hoarseness of her voice, difficulty talking and swallowing, nausea, vomiting, and vertigo related to rapid head turning, tremor of the right arm, and unsteadiness of gait. She reported a weight loss of 28 pounds with a poor diet. Corrected Snellen visual acuities were 20/ 30 OD and 20/ 40 OS. The pupils were equal and reactive to light. Ocular motility examination revealed bilateral weakness of abduction with a primary position 16 prism- diopter esodeviation. Corneal sensation and facial nerve function were intact. The anterior segment examination was unremarkable. Fundus examination showed healthy optic discs and bilateral early retinal pigment epithelial changes in both macular areas. Goldmann visual field assessment was normal OU. Ear, nose, and throat examination revealed a lateral ( cadaveric) position of the vocal cords and bilateral vocal cord palsies. Neurological examination demonstrated impairment of soft palatal movements, brisk upper and lower limb reflexes, facial myokymia, tremor of the right upper J Neuro- Ophthalmol, Vol. 25, No. 2, 2005 101 J Neuro- Ophthalmol, Vol. 25, No. 2, 2005 Hammam et al and lower limbs, and truncal ataxia. An intravenous edrophonium ( Tensilon) test was negative. Cranial magnetic resonance imaging showed several foci of increased T2 signal in the centrum semiovale, peri-trigonal region, and one focus in the left cerebellar hemisphere adjacent to the lateral recess of the fourth ventricle ( Fig. 1). These findings were considered non- specific. Full blood count, peripheral blood film, random blood glucose, urea and electrolytes, liver function tests, and plasma protein electrophoresis were within normal limits. The erythrocyte sedimentation rate was 6 mm/ h. Auto- antibodies including anti- acetylcholine receptor, anti- glycolipid, anti-nuclear screen, complement C3 and C4, extractable nuclear antigens screen for Ro/ La/ Sm/ RNP, anti- nuclear DNA, Crithidia, and rheumatoid factor were all negative. Serum tumor marker studies including alpha- fetoprotein, human chorionic gonadotrophin, carcino- embryonic antigen, and CA 125 were also negative. Cerebrospinal fluid examination showed no evidence of malignant cells, with a cell count of less than 5 nucleated cells/ mm3, an elevated protein level at 1.36 g/ L ( normal range 0.1- 0.5 g/ L), and the presence of oligoclonal bands ( which were absent from the serum). On immunocyto-chemical screening for paraneoplastic antibodies in a validated procedure, microscopical examination of sections of rat cerebral cortex exposed to the patient's serum diluted at 1: 250 and 1: 2500 showed strong evidence of binding to cortical neurons in a pattern consistent with antinuclear ( anti- Hu) reaction ( Fig. 2) with faint neuronal cytoplasmic staining. The presumptive diagnosis was brainstem encephalitis as a consequence of the anti- Hu syndrome. Treatment was administered in the form of intravenous human immunoglobulin ( 0.4 g/ kg body weight, 20 g/ d for 5 days) and intravenous methylprednisolone 1 g/ d for 3 days followed by oral prednisolone 40 mg/ d. This treatment appeared to result in a significant improvement in her speech, swallowing, and double vision. She was maintained on prednisolone 40 mg daily for 4 months. Breast palpation, abdominal ultrasonography, and high- resolution computerized tomography scan of the chest on three occasions did not reveal any abnormality. Neurologically she remained stable and was maintained on prednisolone 30 mg/ d orally for 30 months. Thereafter the dose was tapered to 5 mg on alternate days until the medication was discontinued 36 months after the start of treatment. After an interval of 21 months from the onset of her symptoms, she reported a lump in her right breast and mammography revealed evidence of breast cancer. A right simple mastectomy and axillary clearance disclosed an intraductal and infiltrating ductal breast carcinoma ( grade III). The invasive tumor had metaplastic spindle cell areas, but there was no definite evidence of vascular or lymphatic channel involvement and the deep excision was free of tumor. Microcalcification was present within the tumor. Three of 12 axillary lymph nodes contained metastatic tumor. She received six cycles at 3- week intervals of cyclophosphamide, methotrexate, and 5- fluorouracil. Three years after the mastectomy, she was admitted with pain in the left scapular region and a bone scan showed marked irregular uptake of technetium- 99 throughout the bony skeleton, an appearance consistent with diffuse metastatic disease. An ultrasound scan of the abdomen revealed numerous hypoechoic areas consistent with metastatic deposits. She was referred to the palliative care team and died 4.5 years after the onset of her initial symptoms. FIG. 1. T2- weighted axial magnetic resonance imaging shows several foci of increased signal in the trigones of the lateral ventricles bilaterally ( thin arrows, A) and in the left cerebellar hemisphere adjacent to the lateral recess of the fourth ventricle ( thick arrow, B). 102 © 2005 Lippincott Williams & Wilkins Bilateral Sixth Cranial Nerve Palsies J Neuro- Ophthalmol, Vol. 25, No. 2, 2005 V **. *** » *-. » 4 i • • • •• • . . 4 * •*' ' • - • FIG. 2. Microscopic section of rat cerebral cortex exposed to the patient's serum. The positive reaction of anti- Hu is visualized as a dark stain in the nuclei of neurones within the cerebral cortex with a lighter stain in the cytoplasm. ( Original magnification X200.) DISCUSSION The Hu antigens are a family of nuclear proteins normally expressed in all neurons of the central and peripheral nervous system ( 5). The Hu family antigen was first identified in a report of four patients with subacute sensory neuronopathy associated with lung cancer when a serum antibody reacted with the cytoplasm of neurons in the guinea pig cerebral cortex ( 6). The Hu antigens correspond to a set of proteins of 35 to 40 Kd on Western blot analysis using either neuronal or small cell lung cancer protein extracts. The anti- Hu antibody is a marker not only of sensory neuronopathy but also of encephalomyelitis ( 5). In the diagnosis of paraneoplastic sensory neuropathies, the sensitivity of anti- Hu antibodies is considered to be 82% and the specificity is 99% ( 7). The presence of antibodies against Hu proteins in the serum samples of patients with paraneoplastic syndromes is associated with the destruction of parts of the nervous system and with relative control of growth of the underlying tumor ( 5). These antibodies identify antigens present normally only in the nervous system ( usually in neurons), but for uncertain reasons expressed also in certain tumors. Treatment of the paraneoplastic manifestations may result in a more rapidly growing tumor ( 5). Substantial evidence suggests that in patients with paraneoplastic antibody-positive serology, the neoplasms grow more indolently and are less likely to metastasize than in patients with the same cancer who are not paraneoplastic antibody- positive or who do not have paraneoplastic manifestations. Recent experimental data in mice support these conclusions ( 8). Common findings in paraneoplastic encephalitis are nystagmus and supranuclear vertical gaze palsy, as well as hearing loss, dysarthria, dysphagia, and abnormal respiration. Movement disorders may be prominent ( 9). Other presenting clinical features include seizures, subacute dementia, and personality changes ( limbic encephalitis) ( 10), a combination that may occur alone or with a sensory neuropathy or cerebellar degeneration. Painful paresthesias are the dominant symptom of sensory neuropathy and may progress for days to weeks in all four limbs, the trunk, and sometimes the face. All forms of sensation are affected, resulting in a severe sensory ataxia with loss of deep tendon reflexes ( 11). A review of 22 reported patients with anti- Ri-associated paraneoplastic syndrome showed that four patients had fourth and sixth nerve palsies, and five patients had other ocular motor abnormalities ( 12). Our patient presented with double vision caused by isolated bilateral sixth nerve palsies, followed by impaired talking and swallowing, as well as hoarseness caused by bilateral vocal cord palsies because of affection of the recurrent laryngeal nerves. These clinical features, along with associated nausea, vomiting, vertigo, impaired palatal movements, brisk limbs reflexes, facial myokymia, tremor, and truncal ataxia could not be explained by her non- specific magnetic resonance imaging findings. Magnetic resonance imaging studies of paraneoplastic encephalomyelitis and limbic encephalitis have demonstrated hyperintense temporal T2 signals and contrast-enhanced mesiotemporal signals in Tl- weighted images evolving into temporal lobe atrophy over the course of the disease. Multifocal white matter, perithalamic or striatal lesions, as well as cerebellar atrophy, have been also described. However, these imaging changes are non- specific ( 13,14). The cerebrospinal fluid may be normal but more commonly shows a mild lymphocytosis and a non- specific increase in protein and IgG and IgG index, sometimes with oligoclonal bands ( 14). All therapeutic approaches in the paraneoplastic syndromes must be considered in the light of a possible fluctuating or indolent natural course, spontaneous improvement of neurologic symptoms ( 15), and even spontaneous tumor regression ( 16). Eradication of the cancer is the mainstay of treatment, having a favorable influence on the course of paraneoplastic syndromes. The starting point of good clinical management of patients with a paraneoplastic syndrome is early clinical suspicion. Detection of one of the clinically relevant antibody reactivities proves the paraneoplastic etiology. However, even in the absence of 103 J Neuro- Ophthalmol, Vol. 25, No. 2, 2005 Hammam et al antibodies, a paraneoplastic cause is likely when neurologic symptoms occur within 4 years of cancer diagnosis ( whether before or after), the cerebrospinal fluid shows inflammatory changes, and other causes have been excluded, especially if symptoms regress during effective cancer therapy ( 17). In patients without known cancer but with a high likelihood of paraneoplastic syndromes, detection of the tumor is essential but may be difficult. Because there is a biologically effective immune response against the underlying cancer, the tumor initially may remain small and localized ( 18). If specific antibody reactivities are present, they direct the tumor search to specific organs. If the tumor identified does not fit the known specificity of the paraneoplastic pattern, the possibility of atypical expression of relevant antigens ( 19) or a second malignancy should be considered ( 20). The use of whole- body positron emission tomography with fluorodeoxyglucose has been advocated for early tumor diagnosis in patients with anti- Hu or clinically suspected paraneoplastic syndromes ( 21). REFERENCES 1. Inuzuki T. Autoantibodies in paraneoplastic neurologic syndrome. Am J Med Sci 2000; 319: 217- 26. 2. Dalmau J, Graus F, Rosenblum MK, et al. Anti- Hu- associated paraneoplastic encephalomyelitis/ sensory neuropathy. A clinical study of 71 patients. Medicine 1992; 71: 59- 72. 3. Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurologic syndromes. J Neurol Neurosurg Psychiatry 2004; 75: 1135^ 0. 4. Younger DS, Dalmau J, Inghirami G, et al. Anti- Hu- associated peripheral nerve and muscle microvasculitis. Neurology 1994; 44: 181- 3. 5. Dalmau JO, Posner JB. Paraneoplastic syndromes. Arch Neurol 1999; 56: 405- 8. 6. Wilkinson PC, Zeromski J. Immunofluorescent detection of antibodies against neurones in sensory carcinomatous neuropathy. Brain 1965; 88: 529- 83. 7. Grisold W, Drlicek M. Paraneoplastic neuropathy. Curr Opin Neurol 1999; 12: 617- 25. 8. Carpentier AF, Rosenfeld MR, Delattre JY, et al. DNA vaccination with HuD inhibits growth of neuroblastoma in mice. Clin Cancer Res 1998; 4: 2819- 24. 9. Rawland LP. Paraneoplastic syndromes. Merritt's neurology 20th ed. Philadelphia: Lippincott Williams & Wilkins; 2000: 893- 6. 10. Nath U, Grant R. Neurological paraneoplastic syndromes. J Clin Pathol 1997; 50: 975- 80. 11. Casas Parera I, Fischman D, Paz L, et al. [ Paraneoplastic neuropathy with positive anti Hu]. Medicina ( B Aires) 1998; 58: 197- 201. 12. Sutton IJ, Barnett MH, Watson JD, et al. Paraneoplastic brainstem encephalitis and anti- Ri antibodies. J Neurol 2002; 249: 1597- 8. 13. Benke T, Wagner M, Pallua AK, et al. Long- term cognitive and MRI findings in a patient with paraneoplastic limbic encephalitis. JNeuro- Oncol. 2004; 66: 217- 24. 14. Grant R. What the general neurologist needs to know about the paraneoplastic syndromes. Practical Neurol 2002; 2: 318- 27. 15. Voltz R. Paraneoplastic neurologic syndromes: an update on diagnosis, pathogensis, and therapy. Lancet Neurol 2002; 1: 294- 305. 16. Darnell RB, DeAngelis LM. Regression of small- cell lung carcinoma in patients with paraneoplastic neuronal antibodies. Lancet 1993; 341: 21- 2. 17. Posner JB. Paraneoplastic syndromes. In: Posner JB, ed. Neurologic complications of cancer. Contemporary Neurological series. Philadelphia: FA Davis; 1995: 353- 85. 18. Graus F, Dalmau J, Rene R, et al. Anti- Hu antibodies in patients with small cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol 1997; 15: 2866- 72. 19. Graus F, Keime- Guibert F, Rene R, et al. Anti- Hu associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001; 124: 1138^ 8. 20. Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology 1998; 50: 652- 7. 21. Antoine JC, Cinotti L, Tilikete C, et al. ( 18F) flourodeoxyglucose positron emission tomography in the diagnosis of cancer in patients with paraneoplastic neurologic syndrome and anti- Hu antibodies. Ann Neurol 2000; 48: 105- 8. 104 © 2005 Lippincott Williams & Wilkins |