| Abstract |
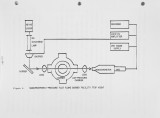
The first results of a comprehensive and systematic experimental program on low-Btu coal-derived syngas/air combustion, underway at The Pennsylvania State University Fuels and Combustion Laboratory, are reported. Gaseous fuels [CH,, CO, H2, NH ] and diluents [CO , N , H O ] were parametrically mixed together, simulating the compositions of near-commercial, coal-derived syngases. The physical [temperature] and chemical [transient and stable species concentrations] microstructure profiles of over 50 non-adiabatic flat Lurgi- and Wellman-Galusha-air flames were determined. From an appliedcombustion research viewpoint, the most significant findings were that: (a) the fuel-bound-nitrogen (FBN=NH«) conversion efficiency, and, therefore, the concentration of NO in the exhaust, maximized at a stoichiometry (equivalence ratio) of 1.2 for both syngas/air flames, and (b) at the same FBN dopinglevel, more NO was produced in Lurgi/air flames than in Wellman-Galusha/air flames. These findings may have an important bearing on: (a) staged-combustion-for-NOX-control-strategies which are based on results from combustion research on flames fueled with individual syngas components that showed the FBN conversion efficiency peaking at stoichiometries of *k 1.0, and (b) coal-derived syngas fuel, and therefore, coal-gasifier, selection, respectively. |