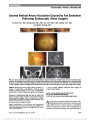
| OCR Text |
Show Suppression of Experimental Autoimmune Optic Neuritis by the Novel Agent Fingolimod Xiaoming An, MD, Takeshi Kezuka, MD, PhD, Yoshihiko Usui, MD, PhD, Yoshimichi Matsunaga, MD, Ryusaku Matsuda, MD, Naoyuki Yamakawa, PhD, Hiroshi Goto, MD, PhD Purpose: Fingolimod is an immunomodulating agent that has been approved for the treatment of multiple sclerosis. Fingolimod-phosphate is an antagonist of sphingosine-1- phosphate receptor and known to act by preventing infiltra-tion of autoreactive lymphocytes into the central nervous system. In this study, we investigated whether fingolimod prevents experimental autoimmune optic neuritis (EAON). Methods: EAON was induced by immunizing C57BL/6 mice with myelin oligodendrocyte glycoprotein-derived peptide 35-55 (MOG-p). After MOG-p immunization, fingolimod was administered intragastrically from day 1 (entire phase study) or from day 9 (effector phase study) until day 35. Visual acuity of the mice was measured using OptoMotry on days 7, 14, 21, 28, and 35 after immunization. On day 35 after immunization, the mice were killed and eyes and entire length of the optic nerves were submitted for histo-pathologic evaluation. Results: In the positive control group, visual acuity decreased markedly from approximately day 14 after immu-nization, reaching a nadir on day 21. In the fingolimod-treated groups in both entire phase and effector phase studies, there was only minimal decline in visual acuity on day 14 after immunization, and mild deterioration on day 21, followed by recovery. Histopathologic study showed that fingolimod given throughout the entire phase or only from the effector phase suppressed murine EAON. Immunohis-tochemical study for neurofilament demonstrated no irregularity of the linear structure of the optic nerve in the fingolimod-treated mice compared with the positive control group. Conclusion: Fingolimod ameliorated EAON even when started after optic neuritis had developed. Further study is warranted to examine whether these findings are applicable to human disease. Journal of Neuro-Ophthalmology 2013;33:143-148 doi: 10.1097/WNO.0b013e31828ea2fc © 2013 by North American Neuro-Ophthalmology Society The pathogenesis of multiple sclerosis (MS)-associated optic neuritis remains incompletely understood but is known to have a strong association with organ-specific auto-immune disease (1). Some central nervous system-specific antigens such as the myelin oligodendrocyte glycoprotein (MOG) and myelin-specific proteolipid protein (PLP) have been shown to induce autoimmune encephalomyelitis and optic neuritis, mimicking the disease spectrum of MS (2,3). MOG antigen, which causes optic neuritis at a high rate, has been shown to be present abundantly within the optic nerve, and inflammatory cells presumably react with the antigen to cause tissue damage. In the mouse model that develops both encephalomyelitis (experimental autoim-mune encephalomyelitis [EAE]) and optic neuritis (experi-mental autoimmune optic neuritis [EAON]), the onset of disease is usually observed on average 13 days after adjuvant immunization with the MOG35-55 peptide (4). When T cells obtained from these mice are injected intraperitone-ally (adoptive immunization) into normal mice, optic neu-ritis also develops in these mice (4). Fingolimod (FTY720; Gilenya, Imusera) is an immuno-modulating agent that has been approved as a new therapeutic drug for MS (5,6). As an antagonist of sphingosine-1- phosphate (S1P) receptor, fingolimod-phosphate is known to act by preventing infiltration of autoreactive lympho-cytes into the central nervous system. Fingolimod amelio-rates EAE probably by reducing infiltration of myelin antigen-specific Th17 and Th1 cells into the central ner-vous system (7). Foster et al (8) reported that fingolimod rapidly blocked ongoing disease processes by inhibiting Department of Ophthalmology, Tokyo Medical University, Tokyo, Japan. The authors report no conflicts of interest. Supported in part by Grant-in-Aid (C) (no. 22591967) for Scientific Research from the Japan Society for the Promotion of Science (Takeshi Kezuka). Address correspondence to: Takeshi Kezuka, MD, PhD, Department of Ophthalmology, Tokyo Medical University, 6-7-1 Nishi-shinjuku, Shinjuku-ku, Tokyo 160-0023, Japan; E-mail: tkezuka@tokyo-med. ac.jp An et al: J Neuro-Ophthalmol 2013; 33: 143-148 143 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. autoimmune T-cell infiltration and directly modulating microvascular and/or glial cells. Moreover, as late-stage rescue therapy, fingolimod reversed blood-brain barrier leakiness and reduced demyelination (7). In this study, we investigated whether fingolimod could prevent EAON and evaluated its clinical potential for the treatment of optic neuritis in humans. METHODS Animals and Anesthesia Six- to 8-week-old female C57BL/6 mice were obtained from Japan Charles River (Ibaraki, Japan). All animal experiments were performed according to the approved guidelines of the Institutional Review Board of Tokyo Medical University, Tokyo, Japan. All animals were treated according to the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. Intraperitoneal injection of a mixture of Nembutal (30 mg/kg) and xylazine hydrochloride (125 mg/kg) was used for anesthesia. Reagents MOG35-55 peptide was synthesized by conventional solid-phase techniques, as described elsewhere (4). Purified Borde-tella pertussis toxin (PTX) was from Sigma Chemical (St. Louis, MO). Complete Freund's adjuvant (CFA) and Mycobacterium tuberculosis strain H37Ra were from Difco (Detroit, MI). Lipopolysaccharide was purchased from Sigma-Aldrich (St. Louis, MO). Fingolimod agent was pro-vided by Novartis Pharma (Basel, Switzerland) and Tanabe Mitsubishi Pharma (Osaka, Japan). EAON Induction and Histopathological Evaluation EAON was induced by the method described by Shao et al (4) with some modifications. The MOG35-55 peptide was diluted to a concentration of 10 mg/mL in phosphate buffer solution (PBS, pH 7.35) containing 50 mL dimethyl sulfox-ide/ 1 mg MOG35-55 peptide and further diluted in PBS and used at a concentration of 200 mg/200 mL per mouse. The MOG35-55 peptide was emulsified at a ratio of 1:1 in CFA containing 5 mg/mL of M. tuberculosis H37RA and used to immunize C57BL/6 mice subcutaneously at the neck region. The mice were also injected intraperitoneally with PTX (1 mg/ 100 mL per mouse) at the same time. The C57BL/6 mice were immunized with MOG35-55 on day 0, followed by per-oral administration of fingolimod from day 1 for entire phase study or from day 9 for effector phase study until day 35. Mice were subjected to euthanasia at 35 days after immunization. The eyes and entire optic nerves were removed and fixed in 10% buffered neutral formalin solutions. Fixed and dehydrated tissue was embedded in methacrylate, and 5-mm sections were cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin. The pathological scores of the eyes lesion were evaluated according to the previous report (4): 0, no lesion; 0.5, slight cell infiltration in optic nerve; 1, moderate cell infiltration in optic nerve; 2, strong cell infiltration in optic nerve; and 3, massive cell infiltration in optic nerve. Immunohistochemistry Immunohistochemical studies for neurofilament (axons) were performed in the entire phase study, on day 0 and day 14 after immunization (n = 5; 5 nonimmunized mice were also measured as normal controls). Mice were subjected to eutha-nasia, and the eyes and optic nerves were fixed in 10% buffered neutral formalin solution. After deparaffinization in xylene and washing in graded ethanol, endogenous perox-idases were quenched with 0.3% H2O2 methanol for 15 mi-nutes. Antigen retrieval was performed using 10M sodium citrate solution (pH 6.0) at 100°C (microwave) for 20 mi-nutes. Rabbit anti-neurofilament monoclonal antibody (1:400; Dako, Tokyo, Japan) was used as primary antibody and applied to the sections for 3 hours at room temperature. Swine polyclonal anti-rabbit IgG (Dako) was used as second-ary antibody. Horseradish peroxidase (HRP)-streptavidin conjugate (Dako) and 3-39-diaminobenzidine tetrahydro-chloride and HRP reaction were used for visualization. Nuclei were stained by hematoxylin. After immunostaining, images were acquired by a light microscope equipped with a digital camera (Olympus BX50, DP70; Olympus, Tokyo, Japan). Fingolimod Administration For the entire phase study, after myelin oligodendrocyte glycoprotein-derived peptide 35-55 (MOG-p) immuniza-tion on day 0, fingolimod at a dose of 0.3 mg/kg was admin-istered by an intragastric route using a probe from day 1, once daily until the end of study on day 35. For the effector phase study, fingolimod was administered intragastrically to MOG-p-immunized mice at a dose of 0.3 mg/kg from day 9, once daily until the end of study on day 35. Positive controls were prepared by intragastric administration of dis-tilled water to MOG-p-immunized mice. Nonimmunized and nontreated mice were used as negative controls. Evaluation of Visual Acuity Visual acuity of the mice was assessed using a recently developed virtual reality optomotor system (OptoMotry; Cerebral Mechanics, Inc., Lethbride, Canada) on days 7, 14, 21, 28, and 35 after immunization (9,10). This system measures optokinetic responses to sine wave gratings of varying spatial frequencies. A contrast sensitivity function is created by identifying the minimum contrast that gener-ates tracking behavior. Statistical Analysis The significance of differences between means was deter-mined using the t test, Mann-Whitney U test, or analysis of 144 An et al: J Neuro-Ophthalmol 2013; 33: 143-148 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. variance followed by Sheffé test. P values less than 0.05 were considered significant. RESULTS Effect on EAON Development Mice were subjected to euthanasia at 35 days after immuni-zation and EAON was evaluated by histopathological examination of the optic nerves. Frequency of EAON was calculated by number of mice with EAON/total number immunized mice. The entire phase study was conducted by immunizing C57BL/6 mice subcutaneously with MOG peptide and then treated with fingolimod from day 1. Eight of 8 positive control mice (100%) (administered distilled water) devel-oped EAON, whereas only 9 of 20 mice (45%) treated with fingolimod developed EAON. The severity of EAON in mice that developed EAON was reduced from a mean score of 2.1 (8 mice in control group) to 0.9 (9 mice in fingolimod-treated group). The difference in EAON scores between fingolimod-treated mice and positive controls was statistically significant (P , 0.001: Mann-Whitney U test) (Fig. 1A). Examples of the inflammatory response within the optic nerve are shown in Figure 1B. Neurofilament immunostaining of the optic nerve on day 14 showed partial irregularity of the linear neurofilament structure in positive control group, which may represent the onset of axon damage. In the fingolimod-treated group, no irregularly stained axons were seen in the optic nerve (Fig. 2). The effector phase study was conducted by immunizing C57BL/6 mice subcutaneously with MOG peptide and then treated with fingolimod from day 9 (effector phase). The severity of EAON was suppressed by administration of fingolimod compared with positive controls. EAON devel-oped in 9 of 10 positive control mice (90%), with mean histopathological score of 2.0 in the 9 mice that developed EAON. In the fingolimod-treated group, EAON developed in 12 of 20 mice (60%), with mean histopathological score of 0.5 in the 12 mice that developed EAON (Fig. 3). The EAON scores between fingolimod-treated mice and positive controls were significantly different (P , 0.001: Mann- Whitney U test). Histologically, infiltration of inflamma-tory cells in the optic nerve was observed in the positive control group (Fig. 3A), whereas there was less cellular infiltration in the fingolimod-treated group (Fig. 3B). Effect on Visual Acuity In the entire phase study, visual acuity on day 14 in positive controls (n = 20) decreased markedly to 0.21 ± 0.11 cycles per degree, whereas visual acuity in the fingolimod-treated group (n = 20) was maintained at 0.36 ± 0.03 cyc/deg. There was a significant difference between two groups (P , 0.001) (Fig. 4). On day 21, visual acuity in the positive controls deteriorated further to 0.08 ± 0.10 cyc/deg, whereas visual acuity in the fingolimod-treated group was reduced only mildly to 0.28 ± 0.11 cyc/deg, also with a significant difference between two groups (P , 0.0001). Thereafter, visual acuity in the fingolimod-treated group began to recover and was restored to a normal level on day 35. In positive controls, visual acuity did improve from day 28 but remained signifi-cantly (P , 0.05) lower than that in the fingolimod-treated group on day 35. In the positive control group, visual acuity decreased gradually from day 14 after immunization and FIG. 1. A. Histopathological scores of optic nerve infiltration in the entire phase study of mice treated with fingolimod vs positive controls. B. Histopathology of optic nerve in EAON mice administered distilled water (left) and fingolimod (right). The positive control shows an inflammatory cellular infiltration (arrowheads), whereas the response is markedly reduced in the mouse administered fingolimod (hematoxylin and eosin, ·100). An et al: J Neuro-Ophthalmol 2013; 33: 143-148 145 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. reached its lowest level on day 21. In the fingolimod-treated group, there was only minimal decline in visual acuity on day 14 and mild deterioration on day 21, followed by recovery to normal. Fingolimod administration before optic neuritis devel-oped was effective in preserving visual acuity in EAON mice. In the effector phase study, visual acuity in the fingolimod-treated group (n = 20) was preserved, with sig-nificant differences compared with positive control group on days 14, 21, 28, and 35 (P , 0.05) (Fig. 5). Results of the effector phase experiment suggest that fingolimod FIG. 2. Immunohistochemical study of neurofilament in the optic nerve in the entire phase study on day 14. A. Normal optic nerve (negative control) shows densely stained neurofilaments as linear structure. B. Optic nerve of an EAON model mouse (positive control) reveals disruption of the linear structure (arrowheads) where cellularity is high. C. Optic nerve of fingolimod-treated EAON model mouse demonstrates no irregularity of linear neurofilament structure and no increased cellularity (neurofilament stain, ·150). FIG. 3. A. Histopathological scores of optic nerve inflammation in effector phase study of mice treated with fingolimod vs positive controls. B. Histopathology of optic nerves in EAON mice administered distilled water (left) and fingolimod (right). In the positive controls, there is an inflammatory cellular response, whereas in the mice treated with fingolimod, the response is markedly reduced (hematoxylin and eosin, ·100). 146 An et al: J Neuro-Ophthalmol 2013; 33: 143-148 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. administration even after optic neuritis develops is poten-tially useful in preserving vision. DISCUSSION In this study, MOG immunization produced EAON in all immunized mice, indicating a high efficiency of MOG antigen in inducing optic neuritis. Oral administration of fingolimod, either from day 1 or from day 9 of MOG immunization, suppressed the development of EAON. Clinically, MOG antigen appears only partially related to MS-associated optic neuritis. In a previous study, we reported data that only 8 of 23 patients (34%) with optic neuritis were MOG antibody seropositive (11). Although MOG-immunized mouse model is an excellent tool for the study of MS, other antigens require further study. Fingolimod is an orally bioavailable compound that has shown efficacy in phase III clinical trials and subsequently was approved for the treatment of MS (12). Fingolimod is phos-phorylated to fingolimod-phosphate in vivo, which resembles naturally occurring S1P. Fingolimod-phosphate, not fingoli-mod itself, is a functional antagonist of S1P1. There are five S1P receptor subtypes, and these receptors are expressed on a wide range of cells involved in many biological processes relevant to MS. S1P1 plays a key role in the immune system, regulating lymphocyte egress from lymphoid tissues into the general circulation. Fingolimod crosses the blood-brain bar-rier and may have direct central nervous system effects, in contrast to other MS therapies that are immunologically tar-geted (12). Köhne et al (13) reported that fingolimod not only exhibits anti-inflammatory properties but also promotes mye-lination in the central nervous system by direct interaction with oligodendrocytes. Using a rat model of EAE, Foster et al (14) found that fingolimod rapidly reduced peripheral lym-phocyte counts with sustained activity at a low subtherapeutic dose. Although blood lymphocyte counts serve as an indicator of fingolimod efficacy, we did not perform these counts, creating limitation of our study. Using a rat optic neuritis model, Rau et al (15) reported that oral administration of fingolimod reduced inflamma-tory cellular infiltration in the optic nerve but had no effect on pattern visual evoked potential (VEP) or the reduced number of retinal ganglion cells. Furthermore, fingolimod treatment did not prevent apoptosis of retinal ganglion cells, and these cells showed persistent activation of apoptotic signaling pathways during fingolimod treatment. In this study, we did not evaluate pattern VEP but used OptoMo-try to measure murine visual acuity. This virtual reality optomotor system developed by Prusky et al (9) quantifies murine visual function in awake animals avoiding adverse effects on electrophysiological recordings caused by nar-cotics (16,17). We found in EAON mice treated with fin-golimod only mild visual disturbance even at day 21 in the entire phase study and almost no vision loss in the effector phase study with subsequent recovery to normal acuity. Potential factors explaining the difference in the effect of fingolimod on visual function in optic neuritis observed in Rau's study and our effector phase study include differences in the model (mouse vs rat) and the dose (our dose was one third the dose) used in the two studies. The basis for vision preservation in the mice treated with fingolimod in our study is unknown. Interestingly, fingolimod treatment subsequent to lysolecithin-induced demyelination in organotypic cerebel-lar slice cultures enhanced remyelination and process exten-sion by mature oligodendrocytes, while increasing microglia numbers and immunoreactivity for the astrocytic marker glial fibrillary acidic protein (18). It may be that fingolimod treat-ment for optic neuritis induces remyelination in EAON mouse model. Our experiment demonstrates that oral administration of fingolimod both from the induction phase and effector phase ameliorated EAON. However, since the entire phase study and effector phase study were conducted at different times, there were some differences in results. The distur-bance of visual acuity in positive controls in the effector phase study (Fig. 5) was milder than that in the entire phase study (Fig. 4). Also, optic neuritis appeared to be slightly milder in the effector phase study (Fig. 3) than in the entire phase study (Fig. 1). These differences could be due to the season of the year, condition of the immunized mice, or other factors. In the effector phase study (Fig. 5), the dis-turbance of visual acuity on the day of starting fingolimod administration (day 9) was similar in the positive control group and fingolimod-treated group. Despite the milder FIG. 4. Effect of oral administration of fingolimod on visual acuity in EAON mice in the entire phase study. FIG. 5. Effect of oral administration of fingolimod on visual acuity in EAON mice in the effector phase study. An et al: J Neuro-Ophthalmol 2013; 33: 143-148 147 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. disturbance of visual acuity in positive controls, visual acuity was significantly preserved in fingolimod-treated mice com-pared with positive controls. Adverse effects of fingolimod have been documented in human studies. Thirteen of 2564 patients (0.5%) treated with fingolimod in the phase III FREEDOMS and TRANSFORMS studies developed macular edema (19). Fingolimod-associated macular edema in the eye seems to be dose dependent and typically resolves upon cessation of therapy. We did not examine the mice treated with fingo-limod for macular edema either clinically or histologically. This study demonstrated both preventive and therapeu-tic effects of fingolimod on EAON and provides proof of concept for the treatment of optic neuritis. It seems that evaluating this medication in human trials is warranted. ACKNOWLEDGMENTS We thank Ms Teresa Nakatani for critical revision of the manuscript. REFERENCES 1. Kezuka T, Usui Y, Goto H. Analysis of the pathogenesis of experimental autoimmune optic neuritis. J Biomed Biotech. 2011;2011:294046. 2. Potter NT, Bigazzi PE. Acute optic neuritis associated with immunization with the CNS myelin proteolipid protein. Invest Ophthalmol Vis Sci. 1992;33:1717-1722. 3. Storch MK, Stefferl A, Brehm U, Weissert R, Wallström E, Kerschensteiner M, Olsson T, Linington C, Lassmann H. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681-694. 4. Shao H, Huang Z, Sun SL, Kaplan HJ, Sun D. Myelin/ oligodendrocyte glycoprotein-specific T-cells induce severe optic neuritis in the C57BL/6 mouse. Invest Ophthalmol Vis Sci. 2004;45:4060-4065. 5. Albert R, Hinterding K, Brinkmann V, Guerini D, Müller- Hartwieg C, Knecht H, Simeon C, Streiff M, Wagner T, Welzenbach K, Zécri F, Zollinger M, Cooke N, Francotte E. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem. 2005;48:5373-5377. 6. Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol. 2009;158:1173-1182. 7. Chiba K, Kataoka H, Seki N, Shimano K, Koyama M, Fukunari A, Sugahara K, Sugita T. Fingolimod (FTY720), sphingosine 1-phosphate receptor modulator, shows superior efficacy as compared with interferon-b in mouse experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2011;11:366-372. 8. Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, Wlachos A, Sobanov J, Kinnunen A, Baumruker T. FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009;19:254-266. 9. Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611-4616. 10. Matsunaga Y, Kezuka T, An X, Fujita K, Matsuyama N, Matsuda R, Usui Y, Yamakawa N, Kuroda M, Goto H. Visual functional and histopathological correlation in experimental autoimmune optic neuritis. Invest Ophthalmol Vis Sci. 2012;53:6964-6971. 11. Kezuka T, Usui Y, Yamakawa N, Matsunaga Y, Matsuda R, Masuda M, Utsumi H, Tanaka K, Goto H. Relationship between NMO-antibody and anti-MOG antibody in optic neuritis. J Neuroophthalmol. 2012;32:107-110. 12. Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91-101. 13. Köhne A, Stettner M, Jangouk P, Dehmel T, Hartung HP, Lehmann HC, Kieseier BC. Fingolimod impedes Schwann cell-mediated myelination: implications for the treatment of immune neuropathies? Effect of fingolimod on Schwann cells. Arch Neurol. 2012;69:1280-1289. 14. Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, Reuschel R, Beerli C, Schwartz M, Billich A. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469-475. 15. Rau CR, Hein K, Sättler MB, Kretzschmar B, Hillgruber C, McRae BL, Diem R, Bähr M. Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis. Am J Pathol. 2011;178:1770-1781. 16. Jehle T, Ehlken D, Wingert K, Feuerstein TJ, Bach M, Lagrèze WA. Influence of narcotics on luminance and frequency modulated visual evoked potentials in rats. Doc Ophthalmol. 2009;118:217-224. 17. Krempler K, Schmeer CW, Isenmann S, Witte OW, Löwel S. Simvastatin improves retinal ganglion cell survival and spatial vision after acute retinal ischemia/reperfusion in mice. Invest Ophthalmol Vis Sci. 2011;52:2606-2618. 18. Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am J Pathol. 2010;176:2682-2694. 19. Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection, and management. Neurology. 2012;78:672-680. 148 An et al: J Neuro-Ophthalmol 2013; 33: 143-148 Basic and Translational Research Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |