| OCR Text |
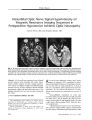
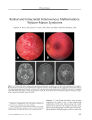
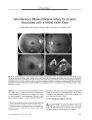
Show ORIGINAL CONTRIBUTION Acute Ophthalmoplegia and Nystagmus in Infants Fed a Thiamine- Deficient Formula: An Epidemic of Wernicke Encephalopathy Anat Kesler, MD, Chaim Stolovitch, MD, Chen Hoffmann, MD, Isaac Avni, MD, and Yair Morad, MD Abstract: In 2003, an epidemic of Wernicke encephalopathy ( WE) developed in Israeli infants fed a thiamine - deficient soy- based formula. Approximately 20 infants were affected out of an estimated 3500 fed the vitamin- deficient formula. The finding of gaze abnormalities in a single infant by neuro- ophthalmologists led to the unraveling of the epidemic. In this report, the findings in three infants are described. Early diagnosis and treatment with parenteral thiamine led to complete neurologic recovery in two infants; in the third infant, delayed diagnosis may have been responsible for severe lingering deficits. This is the first reported epidemic of WE secondary to thiamine- deficient infant formula. Early diagnosis and treatment are critical to avoid persistent neurologic impairment. ( J Neuro- Ophthalmol 2005; 25: 169- 172) ernicke encephalopathy ( WE), a manifestation of thiamine deficiency, is characterized by a triad of gaze abnormalities, mental confusion, and gait ataxia. First described by Carl Wernicke in 1881, these manifestations may develop acutely or subacutely and may occur singly or, more commonly, in various combinations ( 1). In 131 cases described by Harper ( 2), only 16% displayed the full clinical triad, making this condition hard to diagnose. Early recognition of WE is imperative, especially in babies, because the disease may quickly produce devastating neurologic consequences if untreated, yet may be readily reversed with early administration of thiamine ( 1,3,4). In adults, the most prevalent cause of WE is alcoholism accompanied by malnutrition. Other causes Neuro- ophthalmology Unit ( AK) and the Department of Ophthalmology ( AK, CS), Tel Aviv Medical Center, Tel Aviv ( AK, CS); the Department of Radiology, Sheba Medical Center, Tel Hashomer, Israel ( CH); and the Department of Ophthalmology, Assaf Harofeh Medical Center, Zrifm, Israel ( IA, YM). All centers are affiliated with the Sackler School of Medicine, Tel- Aviv University, Tel Aviv, Israel. Address correspondence to Anat Kesler, MD, Neuro- Ophthalmology Unit, Department of Ophthalmology, Tel Aviv Medical Center, 6 Weizmann Street, Tel Aviv, 64239, Israel; E- mail: kesler@ netvision. net. il are parenteral nutrition, excessive vomiting ( in psychiatric disorders or hyperemesis gravidarum), malnutrition alone, cancer, severe infection, gastric bypass surgery, and intestinal malabsorption ( 5- 7). Infantile WE is diagnosed in developing countries primarily in breast- fed infants usually during the second to the fifth month of life ( 1). In developed countries, where infants are usually fed a well- balanced diet, infantile WE is very rare. Although a 1985 Australian study ( 8) demonstrated thiamine deficiency in a high percentage of apparently healthy mothers immediately after birth, no evidence of thiamine deficiency was seen in their newborn infants. This phenomenon can be explained by preferential delivery of thiamine to the fetus during pregnancy. In a follow up of several months, the mothers' thiamine status improved, whereas their breast- fed infants often became thiamine-deficient. The mothers recovered as a result of a thiamine - rich diet, whereas the infants became deficient as a result of the known marginal amount of thiamine in breast milk ( 8- 10). In 2003,20 infants were hospitalized in several Israeli medical centers as a result of an outbreak of a mysterious disease. The infants presented with vomiting, lethargy, loss of consciousness, gaze palsies, and nystagmus. The neuro-ophthalmic manifestations were the clues to the diagnosis of infantile WE ( 11) caused by a thiamine- deficient infant formula. We present the clinical features of three cases. CASE REPORTS Case 1 In November 2003, a six- month- old boy was admitted to the emergency department of " Dana" Children's Hospital, Tel- Aviv, Israel, with apathy, failure to thrive, and daily vomiting episodes for two months. The baby had been given domperidone, an antiemetic drug, to control the vomiting. On examination, abnormal eye movements were noted. To determine if there was a direct relationship to the domperidone, the infant was sent to the pediatric neuro- ophthalmology clinic. J Neuro- Ophthalmol, Vol. 25, No. 3, 2005 169 J Neuro- Ophthalmol, Vol. 25, No. 3, 2005 Kesler et al Examination by two of the authors ( AK, CS) revealed full eye movements with bilateral, symmetric, upbeat nystagmus, equal and reactive pupils, and a normal fundus. This case reminded us of a 16- year- old girl hospitalized for three weeks in the surgical ward of another medical facility 18 months earlier as a result of episodic vomiting of unknown cause. In that case, neuro- ophthalmologic examination had revealed downbeat nystagmus and a confusional state. The final diagnosis was WE confirmed by low levels of vitamin B1. Improvement was shown after intramuscular thiamine injections. In the baby, cranial computed tomography ( CT), magnetic resonance imaging ( MRI), and urine vanilman-delic acid, drawn to rule out neuroblastoma, were normal. Tested to rule out Leigh disease, cerebrospinal fluid lactate was elevated at 3.64 nmol/ L ( normal, 1.1- 2.8 mmol/ L) and plasma lactate was elevated at 4.4 mmol ( normal, 0.5- 2.2 mmol). However, these lactate level elevations proved to be secondary to thiamine deficiency rather than Leigh disease when we found an elevated erythrocyte thiamine-pyrophosphate effect ( TPPE) of 36% ( normal upper limit 14%). Treatment with 50 mg/ kg intramuscular thiamine ( vitamin Bl) per day was started. Thirty- six hours after thiamine administration, there was great improvement in the baby's general condition. Vomiting and irritability disappeared and nystagmus was less severe. The upbeat nystagmus gradually subsided within six weeks. Three months later, he was a healthy child with no neurologic signs, and has remained completely normal after 18 months of follow up. At the time this baby was diagnosed, it became apparent that many infants were being hospitalized all over Israel with similar manifestations. It turned out that they had all been fed the same soybean vegetarian formula manufactured by Humana Milchunion ( Everswinkel, Germany) and distributed in Israel by Heinz Remedia Limited, ( Tel Aviv, Israel). The successful treatment of our case led to an investigation by the Israeli Ministry of Health, which revealed that the levels of thiamine in this formula were barely discernible. The formula was immediately withdrawn from distribution. Case 2 One day after Case 1 was admitted, a six- month- old, previously healthy boy was admitted with lethargy, vomiting, and abdominal tenderness for several days. She had been fed the Remedia formula since birth. Two of the authors ( AK, CS) examined her three days later, finding orthophoria for near, equal and normally reacting pupils, and bilateral abduction deficits of 20%. There was no nystagmus or other ophthalmic abnormalities. Based on the results in Case 1, we suspected infantile WE. Plasma lactate was elevated at 2.4 nmol/ L and TPPE levels were elevated at 31%. Brain MRI was normal. The baby was treated with 50 mg intramuscular thiamine per day, recovering within 24 hours. At a follow- up visit three months later, eye movements were full and the baby was entirely healthy. Case 3 A five- month- old girl fed with Remedia formula since two weeks of age was hospitalized because of apathy and vomiting for one week. The hospital admission occurred two weeks before the admission of Cases 1 and 2 at a time when the WE epidemic had not been recognized. On the day of admission, she experienced several convulsion episodes. The baby had complete vertical and horizontal gaze palsy with preserved convergence. Other aspects of the ophthalmic examination were normal. Plasma and cerebrospinal fluid were elevated ( 6.6 mmol/ L and 4.7 mmol/ L, respectively). On MRI, restricted diffusion was seen in the basal ganglia and T2 high signal in the periaqueductal gray matter ( Fig 1). The initial diagnosis was Leigh disease. Treatment with oral mega- doses of multivitamins, including thiamine, which has been helpful in treating some cases with Leigh disease, was started. A few hours after the initiation of vitamin treatment, the baby became more alert and the vomiting ceased. However, the limitation in ocular ductions persisted. During that time, the baby continued to be fed with the Remedia formula. Two weeks after admission, after the announcement that the Remedia formula was defective, the diagnosis of WE was made. Remedia formula was stopped and thiamine intramuscular injections ( 50 mg per day) were given for six days. TPPE assay done a few hours after the injections began was still slightly high ( 17.6%). After treatment, the baby became more responsive and vigilant; however, on a follow- up visit at the age of 16 months, she was still experiencing convulsions and developmental delay, had complete bilateral abduction palsies, and a nearly complete vertical gaze palsy. Although slight esotropia was noticed occasionally, usually her eyes were aligned in primary gaze and convergence was preserved. DISCUSSION It was eventually estimated that approximately 3500 babies in Israel were fed the thiamine- deficient soybean-based vegetarian formula ( Israel Ministry of Health, unpublished data). After our Case 1 was diagnosed with WE, an estimated 19 babies were so diagnosed. Once the deficiency was discovered, the Israeli Ministry of Health stopped distribution of the formula and warned against its further use. The cause for the low thiamine levels in the new formula is probably human error. This formula, which is 170 © 2005 Lippincott Williams & Wilkins Acute Ophthalmoplegia J Neuro- Ophthalmol, Vol. 25, No. 3, 2005 FIG. 1. Case 3. A. Coronal T2- weighted magnetic resonance image ( MRI) shows high signal in the periaqueductal region ( arrow). B. Axial diffusion- weighted MRI demonstrates hyperintense signal in the putamen region bilaterally ( arrows), consistent with cytotoxic damage. kosher, was distributed only in Israel. An investigation is still being conducted in this matter. To the best of our knowledge, there have been no other outbreaks of vitamin deficiency in infants fed a defective soy- based formula. Early treatment with thiamine, as exemplified by our Cases 1 and 2, was probably critical in reversing the manifestations of WE and preventing permanent neurologic deficits. Our Case 3, in whom the diagnosis was delayed, has persistent ophthalmoplegia and other neurologic deficits. Thiamine is a water- soluble vitamin involved in glucose and lipid metabolism as well as the production of amino acids and glucose- derived neurotransmitters. Dietary thiamine forms thiamine pyrophosphate, which acts as a cofactor in several reactions in the metabolism of carbohydrates. These reactions include the oxidation of pyruvate to acetyl coenzyme A, oxidation of alpha- ketoglutarate in the citric acid cycle, and formation of hexose monophosphates in the pentose phosphate shunt ( 12). Only a small quantity of this vitamin is stored in the body, enough for approximately two weeks. Thiamine depletion will cause a breakdown in glucose metabolism, which is likely to cause muscle and brain damage. The pathophysiology of WE syndrome and other forms of thiamine deficiency remains controversial. Proposed mechanisms include altered cerebral energy metabolism resulting from decreases in pentose phosphate, pyruvate, or acetylcholine pathways, diminished nerve impulse transmission at synapses, and impaired DNA synthesis ( 1,12). The clinical diagnosis of thiamine deficiency is supported by a dietary history suggestive of low thiamine intake and characteristic clinical manifestations. Laboratory diagnosis is complex. Determination of free thiamine in blood plasma does not reliably reflect the thiamine levels in body tissues. Two biochemical tests are used to determine thiamine levels. One measures erythrocyte transketolase activity. The activity of the thiamine- requiring enzyme transketolase appears to provide information as to tissue reserves of thiamine and reflects a direct functional evaluation at the cellular level. The other test, the one we used, is the TPPE, which examines the effect on transketolase activity of adding thiamine pyrophosphate to the reaction mixture and comparing it with transketolase activity without the addition of thiamine pyrophosphate. TPPE is expressed in percentage stimulation compared with baseline, the normal range being 0% to 14%. A value of 15% to 24% indicates marginal thiamine deficiency and > 25% indicates severe deficiency ( 7,13). Before the epidemic of WE was recognized in Israel, Case 3 was believed to have Leigh disease or subacute necrotizing encephalomyelopathy, a neurodegenerative disease caused by defects in mitochondrial energy generation, including pyruvate dehydrogenase complex and cytochrome C oxidase deficiency ( 14). Leigh disease is characterized by the subacute onset of psychomotor retardation, hypotonia, ataxia weakness, gaze palsy, nystagmus, seizures, dysphagia, and lactic acidosis. These features are also found in WE, hence the ease of confusing the two diagnoses. Although blood and cerebrospinal fluid lactate are elevated in both Leigh disease and WE, the erythrocyte transketolase activity and TPPE are normal in Leigh disease. When Leigh disease was suspected in Case 3, the infant was treated with oral multivitamins because thiamine treatment has been reported to be effective in some cases with Leigh disease ( 15). However, only after ceasing the defective formula and starting treatment with intramuscular thiamine injections did substantial clinical improvement begin. In their review of pediatric childhood WE, Vascon-celos et al ( 5) emphasized that WE may be fatal if untreated. Although all the children were ill before diagnosis, some with severe gastrointestinal problems, only 18 of 31 ( 58%) 171 J Neuro- Ophthalmol, Vol. 25, No. 3, 2005 Kesler et al were diagnosed and treated; 13 ( 42%) were diagnosed by post- mortem examination only ( 5). Although approximately 3500 babies were fed the defective formula in Israel, only 20 became ill with WE. Two explanations can be given as to why such a small percentage was affected. First, the mothers of affected children may have had particularly low thiamine levels, making their infants vulnerable to the thiamine- deficient formula. Vitamin B is transferred across the placenta during pregnancy, and therefore extremely low maternal thiamine blood levels can lead to thiamine deficiency in their newborn infants ( 8). A second, and more likely, explanation is that the affected children may have had a genetic predisposition to clinical thiamine deficiency. Sir Archibald Garrod in 1928 expanded his concept of inborn errors of metabolism to include inborn predispositions to metabolic disorders, suggesting that inborn predispositions resulted from abnormalities in specific proteins. Unlike inborn errors, inborn predispositions are likely to be clinically silent unless the person with a predisposition faces an appropriate stress ( 4,8). In 1977, Blass et al ( 16) found abnormalities in a thiamine - dependent transketolase in four cases with WE. The abnormalities in the enzyme appeared to be genetic rather than a consequence of the disease inasmuch as they persisted in fibroblasts through more than 20 generations of culture medium containing excess thiamine and no ethanol ( 16). These abnormalities made the cases susceptible to thiamine deficiency and prone to develop WE. The genetic predisposition hypothesis was later validated in a subgroup of alcohol abusers who developed WE and had abnormally low activity levels of transketolase ( 17). It is therefore possible that the babies who develop WE after marginal thiamine intake are genetically predisposed because of abnormal transketolase. We recommend that WE be considered in cases with eye movement disorders at any age, particularly if there are other clinical features of thiamine deficiency In such cases, laboratory tests such as TPPE should be performed, followed by the administration of thiamine parenterally at 50 mg per day until the TPPE results are known. REFERENCES 1. Victor M, Ropper AH. Diseases of the nervous system due to nutritional deficiency. In: Victor M, Ropper AH, eds. Adams and Victor Principles of Neurology, 7th ed. New York: McGraw- Hill, 2001: 1205- 51. 2. Harper CG, Giles M, Finlay Jones R. Clinical signs in the Wernicke Korsakoff complex: A retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 1986; 49: 341- 5. 3. Sharma S, Sumich PM, Francis IC, et al. Wernicke's encephalopathy presenting with upbeating nystagmus. J Clin Neurosci 2002; 9: 476- 8. 4. Cogan DG, Witt ED, Goldman- Rakic PS. Ocular signs in thiamine-deficient monkeys and in Wernicke's disease in humans. Arch Ophthalmol 1985; 103: 1212- 20. 5. Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke's encephalopathy. Pediatr Neurol 1999; 20: 289- 94. 6. Reuler JB, Girard DE, Cooney TG. Wernicke encephalopathy. NEngl J Med 1985; 312: 1035- 9. 7. Blass JP, Gibson GE. Abnormality of a thiamine- requiring enzyme in patients with Wernicke- Korsakoff syndrome. N Engl J Med 1977; 297: 1367- 70. 8. Jeffrey HE, McCleary BV, Hensley WJ, et al. Thiamine deficiency- A neglected problem of infants and mothers- Possible relationships to sudden infant death syndrome. AustNZ JObstet Gynaecol 1985; 25: 198- 202. 9. Slobody LB, Willner MM, Mestern J. Comparison of vitamin Bi levels in mothers and their newborn infants. Am JDis Child 1949; 77: 736^ 10. 10. Holt LE Jr, Nemir RL, Snyderman SE, et al. The thiamine requirement of the normal infant. JNutr 1949; 37: 53- 66. 11. Fattal- Valevski A, Kesler A, Sela BA, et al. Outbreak of life-threatening thiamine deficiency in infants in Israel caused by a defective soy- based formula. Pediatrics 2005; 115: e233- 8. 12. Manzo L, Locatelli C, Candura SM, et al. Nutrition and alcohol neurotoxicity. Neurotoxicology 1994; 15: 555- 65. 13. Doolman R, Dinbar A, Sela BA. Improved measurement of transketolase activity in the assessment of ' TPP effect.' Eur J Clin Chem Clin Biochem 1995; 33: 445- 6. 14. Rahman S, Blok RB, Dahl HH, et al. Leigh syndrome: Clinical features and biochemical and DNA abnormalities. Ann Neurol 1996; 39: 343- 51. 15. Di Rocco M. Lamba LD, Minniti G, et al. Outcome of thiamine treatment in a child with Leigh disease due to thiamine- responsive pyruvate dehydrogenase deficiency. Eur J Paediatr Neurol 2000; 4: 115- 7. 16. Blass JP, Gibson GE. Abnormality of a thiamine- requiring enzyme inpatients with Wernicke- Korsakoff syndrome. N Engl J Med 1977; 297: 1367- 70. 17. Heap LC, Pratt OE, Ward RJ, et al. Individual susceptibility to Wernicke Korsakoff syndrome and alcoholism induced cognitive deficit: Impaired thiamine utilization found in alcoholics and alcohol abusers. Psychiatr Genet 2002; 12: 217- 24. 172 © 2005 Lippincott Williams & Wilkins |