| OCR Text |
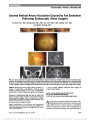
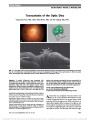
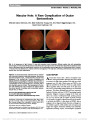
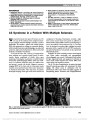
Show Radiation Optic Neuropathy After Proton Beam Therapy for Optic Nerve Sheath Meningioma Jamal D. Siddiqui, MD, Jay S. Loeffler, MD, Marjorie A. Murphy, MD Abstract: A 63-year-old woman with a 1-month history of blurred vision in the right eye was found to have a right optic nerve sheath meningioma. She was treated with fractionated proton beam therapy using a total dose of 50.4 cobalt gray equivalent (CGE) in 1.8 CGE fractions, with subsequent improvement in vision. Twenty-seven months later, the patient reported a 6-week history of progressive blurred vision in her right eye. Magnetic resonance imaging revealed enhancement of the right optic nerve consistent with radiation optic neuropathy (RON). We are unaware of any previous reports of RON when radiotherapy doses fall within the current recommended guidelines of ,55 CGE fractionated into daily doses ,2 CGE. Journal of Neuro-Ophthalmology 2013;33:165-168 doi: 10.1097/WNO.0b013e31828292b8 © 2013 by North American Neuro-Ophthalmology Society Optic nerve sheath meningioma (ONSM) is a benign neoplasm arising from meningoepithelial cap cells of arachnoid villi encapsulating the optic nerve or, less com-monly, from intracranial extension of tumor through the optic canal. ONSM frequently leads to painless progressive visual loss due to optic nerve, tract, or chiasmal compression. Fractionated radiation therapy has been established as the preferred treatment modality for this neoplasm (1). Radiation optic neuropathy (RON) is an uncommon but devastating adverse complication of radiation therapy directed at or adjacent to the visual pathways. Kline et al (2) proposed diagnostic criteria for RON, including acute visual loss, visual field defects consistent with optic nerve or chiasmal injury, lack of optic disc edema, absence of visual pathway compression on neuroimaging, and onset usually within 3 years of radiation. While a variety of factors may contribute to RON, it is clear that, with external beam radiotherapy, total dose and fraction size are important. Our patient with ONSM developed RON after receiving proton beam therapy. We are unaware of previous reports of RON for ONSM when current recommended treatment guidelines are followed. CASE REPORT A 63-year-old woman presented with a 1-month history of blurred vision in the right eye. Visual acuity was 20/40, right eye, and 20/25, left eye. There was a right relative afferent pupillary defect, diminished color vision in the right eye, and right optic disc pallor. The right visual field showed generalized depression (Fig. 1A). Her medical history included treatment for hypertension and hyperlipidemia. Medical evaluation, including testing for sarcoidosis, was unremarkable. Magnetic resonance imag-ing (MRI) showed tram-track enhancement of the right optic nerve consistent with ONSM (Fig. 2). Three months after diagnosis, the patient was treated with fractionated proton beam therapy with a total dose of 50.4 cobalt gray equivalent (CGE) in 1.8 CGE fractions. Three months later, the visual acuity was 20/20, right eye, and pupillary reactions were normal. By 17 months, visual fields were markedly improved (Fig. 1B). Twenty-seven months after proton beam therapy, the patient reported a 6-week history of progressive blurred vision in the right eye, particularly temporally. Visual acuity was 20/40, right eye, and 20/20, left eye, with a right relative afferent pupillary defect and no change in the optic disc pallor. The right visual field showed primarily temporal loss (Fig. 1C). MRI demonstrated enhancement of the right optic nerve approaching the chiasm (Fig. 3). The patient was diagnosed with RON in the right eye and treated with 1 g of methylprednisolone intravenously daily for 5 days. Department of Ophthalmology (JDS), Rhode Island Hospital, Prov-idence, Rhode Island; Department of Radiation Oncology (JSL), Massachusetts General Hospital, Boston, Massachusetts; and Department of Ophthalmology (MAM), Providence Veterans Affairs Medical Center, Providence, Rhode Island. The authors report no conflicts of interest. Address correspondence to Marjorie A. Murphy, MD, VA at Eagle Square, 589/623 Atwells Ave., Providence, RI 02909; E-mail: margiemurphy@cox.net Siddiqui et al: J Neuro-Ophthalmol 2013; 33: 165-168 165 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Over 11 months of follow-up, the patient's visual acuity and field in the right eye has remained stable (Fig. 1D). DISCUSSION Current treatment guidelines to avoid RON include total dose of 50-55 Gy fractionated into individual doses of less than 2 Gy. Andrews et al (3) reported no cases of RON from 33 optic nerves irradiated with 50.4 Gy. Metellus et al (4) also reported no cases of RON in 9 patients with ONSM treated with total doses of 50.4 Gy. Abouaf et al (5) did not report RON occurring in 10 patients treated for ONSM, with total doses ranging from 50 to 64 Gy. When these recommendations are exceeded, RON has been reported in treating head and neck tumors in close proximity to the visual pathways. Parsons et al (6) found no cases of RON in 106 optic nerves that received doses less than 59 Gy, but reported 17 cases of RON in optic nerves that received higher doses. Bhandare et al (7) described RON in 32 optic nerves receiving mean total doses of 67 Gy. FIG. 1. Automated visual fields. A. Before proton beam therapy. Results after treatment at 17 months (B), 27 months (C), and 38 months (D). 166 Siddiqui et al: J Neuro-Ophthalmol 2013; 33: 165-168 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Our patient was treated with proton beam therapy rather than conventional external beam radiation therapy using photons. Proton therapy has the advantage of a finite energy path, limiting radiation exposure to adjacent structures (8). For ONSM, it eliminates potential damage to the contra-lateral optic nerve and chiasm. Protons are highly adaptable to irregular targets of irregular shape and are believed to have a high relative biological effectiveness compared with photons. For proton beam therapy, radiation doses are measured in CGE (proton Gy · 1.1). There have been no reports of RON following proton beam treatment for ONSM or head and neck tumors when doses fall within current recommendations (9-12). Lessel (13) reported that RON may occur at radiation dosages lower than those believed safe in patients with Cushing syndrome, pituitary tumor, diabetes mellitus, or those receiving chemotherapy. None of these factors was present in our patient. A variety of treatment options have been described for RON, including corticosteroids (14-16), anticoagu-lation (17,18), hyperbaric oxygen (14-17,19), and anti- vascular endothelial growth factor agents (20,21). None is of proven efficacy. We were gratified but have no good explanation as to why a 5-day course of high-dose intra-venous steroids led to stabilization of our patient's visual function. In summary, although fractionated radiotherapy remains the treatment of choice for ONSM, our report illustrates that RON may occur even at recommended radiation doses believed to be safe. REFERENCES 1. Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890-899. 2. Kline LB, Kim JY, Ceballos R. Radiation optic neuropathy. Ophthalmology. 1985;92:1118-1126. 3. Andrews DW, Faroozan R, Yang BP, Hudes RS, Werner- Wasik M, Kim SM, Sergott RC, Savino PJ, Shields J, Shields C, Downes MB, Simeone FA, Goldman HW, Curran WJ. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890-904. 4. Metellus P, Kapoor S, Kharkar S, Batra S, Jackson J, Kleinberg L, Miller NR, Rigamonti D. Fractionated conformal radiotherapy for management of optic nerve sheath meningiomas: long-term outcomes of tumor control and visual FIG. 2. A. Contrasted T1 axial magnetic resonance imaging shows tram-track enhancement of right optic nerve (arrow) consistent with optic nerve sheath meningioma. B. More rostral image shows no intracranial involvement. FIG. 3. A. Contrast-enhanced T1 axial magnetic resonance imaging reveals no change in size of right optic nerve sheath meningioma (arrow). B. There is enhancement of the prechiasmal portion of the right optic nerve (arrowhead) consistent with radiation optic neuropathy. Siddiqui et al: J Neuro-Ophthalmol 2013; 33: 165-168 167 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. function at a single institution. Int J Radiat Oncol Biol Phys. 2011;80:185-192. 5. Abouaf L, Girard N, Lefort T, D'Hombres A, Tilikete C, Vighetto A, Mornex F. Standard-fractionated radiotherapy for optic nerve sheath meningioma: visual outcome is predicted by mean eye dose. Int J Radiat Oncol Biol Phys. 2012;82:1268- 1277. 6. Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755-763. 7. Bhandare N, Monroe AT, Morris CG, Bhatti MT, Mendenhall WM. Does altered fractionation influence the risk of radiation-induced optic neuropathy? Int J Radiat Oncol Biol Phys. 2005;62:1070-1077. 8. Hug EB. Review of skull base chordomas: prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg Focus. 2001;10:e11. 9. Weber DC, Lomax AJ, Rutz HP, Stadelmann O, Egger E, Timmermann B, Pedroni ES, Verwey J, Miralbell R, Goitein G. Spot-scanning proton radiation therapy for recurrent, residual or untreated intracranial meningiomas. Radiother Oncol. 2004;71:251-258. 10. Wenkel E, Thornton AF, Finkelstein D, Adams J, Lyons S, De La Monte S, Ojeman RG, Munzenrider JE. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363-1370. 11. Kim J, Munzenrider J, Maas A, Finkelstein D, Liebsch N, Hug E, Suit H, Smith A, Goitein M. Optic neuropathy following combined proton and photon radiotherapy for base of skull tumors. Int J Radiat Oncol Biol Phys. 1997;39:272. 12. Arvold ND, Lessell S, Bussiere M, Beaudette K, Rizzo JF, Loeffler JS, Shih HA. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75:1166-1172. 13. Lessell S. Friendly fire: neurogenic visual loss from radiation therapy. J Neuroophthalmol. 2004;24:243-250. 14. Roden D, Bosley TM, Fowble B, Clark J, Savino PJ, Sergott RC, Schatz NJ. Delayed radiation injury to the retrobulbar optic nerves and chiasm: clinical syndrome and treatment with hyperbaric oxygen and corticosteroids. Ophthalmology. 1990;97:346-351. 15. Borruat F-X, Schatz NJ, Glaser JS, Matos L, Feuer W. Radiation optic neuropathy: report of cases, role of hyperbaric oxygen therapy and literature review. J Neuroophthalmol. 1996;16:255-266. 16. Boschetti M, De Lucchi M, Giusti M, Spena C, Corallo G, Goglia U, Ceresola E, Resmini E, Vera L, Minuto F, Ferone D. Partial visual recovery from radiation-induced optic neuropathy after hyperbaric oxygen therapy in a patient with Cushing disease. Eur J Endocrinol. 2006;154:813-818. 17. Danesh-Meyer HV, Savino PJ, Sergott RC. Visual loss despite anticoagulation in radiation induced optic neuropathy. Clin Experiment Ophthalmol. 2004;32:333-335. 18. Barbarosa AP, Cavalho D, Marrques L, Monteiro M, Castro Neves A, Machado Carvalho A, Cruz J, Medina JL. Inefficiency of the anticoagulant therapy in the regression of the radiation-induced optic neuropathy in Cushing's disease. J Endocrinol Invest. 1999;22:301-305. 19. Guy J, Schatz NJ. Hyperbaric oxygen in the treatment of radiation-induced optic neuropathy. Ophthalmology. 1986;93:1083-1088. 20. Finger PT. Anti-VEGF bevacizumab (Avastin) for radiation optic neuropathy. Am J Ophthalmol. 2007;143:335-338. 21. Finger PT, Chin KJ. Antivascular endothelial growth factor bevacizumab for radiation optic neuropathy: secondary to plaque radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:789-798. 168 Siddiqui et al: J Neuro-Ophthalmol 2013; 33: 165-168 Clinical Observation Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |