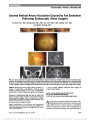
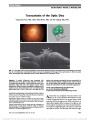
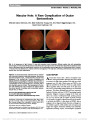
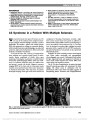
| OCR Text |
Show Dural Puncture-Induced Intracranial Hypotension Causing Diplopia Padmaja Sudhakar, MD, Jonathan D. Trobe, MD, Jeffrey Wesolowski, MD Background: Diplopia that occurs after an epidural spinal catheter has been placed for pain control has been attributed to sixth nerve palsy nerve palsy induced by intracranial hypo-tension. There is sparse information about the factors that confound diagnosis in this setting. Methods: Review of 6 cases examined over a period of 5 years at a single tertiary care medical center. Results: Six confounders to diagnosis were identified: 1) lack of awareness that an epidural spinal catheter was or had been in place; 2) delayed reporting of diplopia; 3) mild or inapparent ductional deficits; 4) lack of postural headache; 5) clinical features that suggested an alternative diagnosis; 6) neuroim-aging features that did not allow exclusion of pachymeningitis. Conclusion: Clinicians should be aware of features that confound a diagnosis of dural puncture-induced intracranial hypotension as a cause of diplopia in the post-operative period when an epidural pain control system is or has been deployed. If these confounders are recognized and the correct diagnosis is reached, radiologists will be less likely to diagnose pachy-meningitis and clinicians will be able to avoid lumbar puncture, which may exacerbate the condition. Journal of Neuro-Ophthalmology 2013;33:106-112 doi: 10.1097/WNO.0b013e318273bff4 © 2013 by North American Neuro-Ophthalmology Society Indwelling spinal epidural catheters offer a generally safe and effective method of providing continuous intraoper-ative and postoperative analgesia. But considerable skill is involved in proper placement of the catheter. Even in skilled hands, accidental dural puncture may occur, estimated at a frequency of 0.19%-3.6% (1-4). The most common enduring complication of accidental dural puncture is "postdural puncture headache" (PDPH), reli-ably distinguished from other headache only by being more intense upon sitting or standing. Its incidence after dural punc-ture is estimated at 50%-80% (5,6), and higher when cutting or larger gauge needles are used (7). PDPH is attributed to intracranial hypotension (IH) owing to leakage of cerebrospinal fluid through the dural hole. Because the epidural needles are usually 18-gauge, wider than the 22-gauge needles recommen-ded for lumbar puncture (7), PDPH is likely to be more com-mon after accidental dural puncture than after lumbar puncture. Brain imaging often displays one or more of the following signs of IH: subdural fluid collection, dural thickening and excessive contrast enhancement, engorged dural venous sinuses or change in sinus configuration, pituitary gland enlargement, and downward displacement of the brainstem (8-16). The most common neurologic sign associated with PDPH is diplopia, usually caused by sixth nerve palsy, and estimated to occur in no more than 0.25% of dural punctures. The palsy is attributed to tension on the nerve as the brainstem sinks in IH (17). Because epidural pain control systems are used so com-monly and IH is relatively rare in this setting, clinicians are apt to overlook IH as the cause of postoperative diplopia, particu-larly if the ophthalmologic findings are subtle or appear well after the epidural pain catheter has been removed, or if the patient fails to report the postural nature of the headache. If radiologists are not alerted that IH is a consideration, they may not look for its imaging features or incorrectly attribute these features to inflammation. We describe 6 cases of sixth nerve palsy following presumed dural puncture in epidural pain control systems to highlight confounding features in the diagnosis of IH in this setting. Recognizing these confounders, clinicians and radiologists should be able to reject alternative diagnoses and avoid diagnostic lumbar puncture, which could exacerbate the condition. METHODS The January 2006 to December 2011 records of the Neuro- Ophthalmology and Ophthalmology Inpatient Consultation Departments of Ophthalmology and Visual Science (PS, JDT), Neurology (JDT), and Radiology (Neuroradiology) (JW), University of Michigan, Ann Arbor, Michigan. The authors report no conflicts of interest. Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the full text and PDF versions of this article on the journal's Web site (www. jneuro-ophthalmology.com). Address correspondence to Jonathan D. Trobe, MD, Department of Ophthalmology and Visual Sciences, W. K. Kellogg Eye Center, 1000 Wall Street, Ann Arbor, MI 48105-0714; E-mail: jdtrobe@med.umich.edu 106 Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Services of the University of Michigan Medical System were searched for patients who had been diagnosed with sixth nerve palsy ultimately attributed to IH associated with an epidural pain control system. At least one characteristic magnetic resonance imaging (MRI) feature of IH had to be present. Six patients met entry criteria and are the basis of this report. For the purposes of this study, their brain MRIs were re-reviewed by a single neuroradiologist (J.W.), who graded the presence of 4 brain imaging signs of IH: subdural fluid collection, excessively enhancing and thickened pachymeninges, enlarged pituitary gland, and downward displacement of the brainstem (9-16). Each sign was assigned a grade, as follows: 0 = absent, 1 = mild, and 2 = marked. We elected not to include measurement of the size of the dural sinuses because of great normal variability. Investigational review board permission was granted. CASE REPORTS Case 1 A 22-year-old man with refractory inflammatory bowel disease reported binocular horizontal diplopia but no headache 9 days after undergoing subtotal colectomy and ileostomy followed by gastrojejunostomy tube placement. Postoperatively, he received an epidural bupivacaine infusion catheter for pain management that was removed 4 days later. He had been treated chronically with prednisone and tacrolimus (Table 1). He had no other relevant medical history and denied other neuro-ophthalmologic symptoms. Bedside examina-tion disclosed normal visual function. The ocular adnexal examination was normal, but the conjunctiva was hyper-emic in both eyes without discharge. There was mild limitation of abduction of the right eye with a slightly incomitant esodeviation. Other aspects of the neuro-ophthalmologic examination were normal. In the setting of inflammatory bowel disease, the ophthalmologic findings raised a suspicion of orbital inflam-matory disease. Brain and orbit MRI showed grade 2 pachymeningeal enhancement and thickening but no other signs of IH (Fig. 1). Because the interpreting neuroradiologist was not informed that an epidural pain catheter had previ-ously been in place, the possibility of an inflammatory or neoplastic cause of the meningeal findings was considered. Following an unsuccessful bedside lumbar puncture, a fluoro-scopically guided lumbar puncture yielded fluid at such a slow rate that an opening pressure could not be measured. The cerebrospinal constituents were normal. Corticosteroid eyedrops were administered for presumed conjunctival inflammation. Within 2 weeks, the diplopia resolved and headache subsided within the next few weeks. Over the next 2 years, no new systemic or neuro-ophthalmologic abnormalities developed. Case 2 A 38-year-old woman reported binocular horizontal diplo-pia 6 days after undergoing open biliary cystectomy and cholecystectomy for a recently diagnosed liver cyst. She also described postoperative headache and neck stiffness. As she was bed confined, she could not report whether the headache was postural. A pain management epidural catheter placed immediately after surgery was removed on the fifth post-operative day, and one day later she reported diplopia. In addition, the patient experienced night sweats and a 15-lb weight loss over the previous 6 months (Table 1). Visual acuity and confrontation visual fields were normal, as were pupil size and reactivity. The left eye had limited abduction while other ductions were normal. The patient had a 20 prism-diopter esotropia in primary gaze, increasing to 30 prism diopters in left gaze and decreasing to 14 prism-diopters in right gaze. All other aspects of the neuro-ophthalmologic examination were normal. Brain MRI showed grade 1 pachymeningeal enhancement and grade 1 pituitary enlargement (Fig. 2). Grade 1 bilateral subdural fluid collections, overlooked initially, were detected on later review. In view of the reported weight loss and night sweats, the radiologist could not exclude infectious or neo-plastic meningitis as the cause of the MRI findings. Before lumbar puncture, as the patient became ambu-latory, she reported that her headache was postural. With that information, clinicians thought of the epidural pain catheter, presumptively diagnosed IH, and canceled the lumbar puncture. Two weeks later, her headache resolved, but the left abduction deficit was still present. Five weeks later, the deficit had improved, but she still had esotropia in primary and left gaze. Case 3 A 21-year-old woman received epidural analgesia for partu-rition. On the first postpartum day, she developed a severe headache, seizures, and depressed consciousness (Table 1). Brain computed tomography (CT) revealed a large right subdural hematoma with midline shift requiring emergent craniotomy. Several weeks later, she reported having binocular horizontal diplopia of an uncertain duration. All aspects of the neuro-ophthalmologic examination were normal except for a 14 prism-diopters esotropia in left gaze, consistent with a left sixth nerve palsy. Brain MRI showed grade 1 pachymeningeal enhancement and thick-ening, grade 2 pituitary gland enlargement, and grade 2 downward displacement of the brainstem consistent with IH (Fig. 3). The patient underwent epidural blood patching and the ocular motor findings gradually resolved. Case 4 A 36-year-old man presented with horizontal binocular diplopia. Fourteen months earlier, he had undergone excision Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 107 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. TABLE 1. Clinical and neuroimaging features of 6 patients with diplopia and intracranial hypotension Case 1 2 3 4 5 6 Age/sex 22/M 38/F 21/F 36/M 71/F 35/F Setting Colectomy for refractory inflammatory bowel disease Biliary cystectomy for liver cyst Normal parturition with epidural analgesia Vertebrectomy for metastatic skin melanoma, Jackson-Pratt drain Reduction pneumoplasty for severe emphysema Ileocecal revision for Crohn disease Latency to reporting diplopia 9 days after surgery 5 days after surgery Uncertain because of clouded sensorium 10 days after surgery 14 days after surgery 6 days after surgery Headache No Yes, later determined to be postural Uncertain (clouded consciousness) Yes, postural No Yes, postural Brain MRI Pachymeningeal enhancement and thickening Subdural fluid collection (initially overlooked) Pachymeningeal enhancement Pachymeningeal enhancement and thickening Pachymeningeal enhancement Pachymeningeal enhancement and thickening Pachymeningeal enhancement Pituitary gland enlargement Mild pituitary enlargement Enlarged pituitary gland Pituitary gland enlargement Downward displacement of cerebellar tonsils Mild downward displacement of brainstem Mild downward displacement of brainstem Lumbar puncture Trickle Not done Not done No fluid emerged (dry tap)from lumbar space; normal CSF from cervical puncture No fluid obtained due to difficulty positioning patient (dry tap) Not done Clinical confounders No headache Diplopia reported 1 day after catheter removed Following parturition, subdural hemorrhage requiring evacuation Known metastatic melanoma No headache Diplopia reported 4 days after epidural catheter removed Diplopia reported 5 days after catheter removed - Diplopia reported several weeks after delivery Diplopia reported 10 days after surgery Diplopia reported 14 days after surgery Normal ocular ductions with small comitant esotropia in side gaze Red eyes in setting of inflammatory bowel disease suggested orbitopathy - - - Nearly comitant esodeviation attributed to fusion loss from pain medication Spinal MRI fluid collection suggested abscess Minimal ductional deficit - - - - - MRI, magnetic resonance imaging. 108 Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. of a malignant melanoma on his upper back. Right axillary lymph node dissection was positive (Table 1). One year later he developed low back, left hip, anterior thigh, and knee pain. Brain MRI was normal, but MRI of the spine showed T1, T5, L2, and L5 vertebral body lesions suggestive of metastatic melanoma. He underwent L2 partial vertebrectomy, removal of an intraspinal mass, confirmed pathologically as melanoma, and L1-L3 posterior segmental fusion. On the 10th postoperative day, he reported new binocular horizontal diplopia worse on left gaze and associated with headache exacerbated by sitting up. Neuro-ophthalmological examination was normal apart from mild limitation of abduction of the left eye. He had 6 prism-diopters of esophoria in the primary gaze position, increasing to 18 prism-diopters of esotropia on left gaze and 4 prism-diopters of esophoria on right gaze. Brain MRI showed grade 2 pachymeningeal enhancement and thickening and grade 2 downward displacement of the brainstem (See Supplemental Digital Content, Figure 1, http://links.lww.com/WNO/A53). Given his history, these findings were interpreted as suggestive of meningeal spread of melanoma. Lumbar puncture yielded no cerebrospinal fluid but fluid was obtained with cervical puncture and had normal constituents. Chest, abdomen, and pelvis CT showed no metastatic lesions. After these studies had been performed, it became apparent that a Jackson-Pratt drain had been left in the epidural space for 4 days postoperatively. Brain MRI findings were now reinterpreted as consistent with IH. One month later, the patient's headache and neuro-ophthalmologic find-ings had resolved. Case 5 A 71-year-old woman reported the sudden onset of horizontal binocular diplopia on the 14th day following bilateral reduction pneumoplasty for severe emphysema. An epidural pain-control catheter had been in place since the surgery. The patient denied headache, although she had been largely bed-confined. There were no other pertinent symptoms (Table 1). Bedside neuro-ophthalmic examination disclosed an alert patient with no abnormalities apart from ocular motility and alignment. Ductions were full, but abducting saccades were slower than adducting saccades in both eyes. She described slightly wider separation of diplopic images on side gaze than in primary gaze, that appeared equal in right gaze and left gaze. Measurement of alignment indicated 8 prism-diopters of esotropia in primary gaze position and slightly greater esodeviation in right and left gaze. These FIG. 1. Case 1. Postcontrast T1 axial magnetic resonance imaging demonstrates grade 2 pachymeningeal thickening and enhancement (arrows). FIG. 2. Case 2. A. Postcontrast T1 coronal magnetic resonance imaging discloses grade 1 pachymeningeal enhancement (arrows) and grade 1 subdural effusions (arrowheads). B. Precontrast T1 sagittal image shows grade 1 prominence of the pituitary gland (arrow). Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 109 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. findings were attributed to a break in fusion because of narcotic medication. Brain MRI showed grade 1 pachymeningeal enhancement but no other findings to suggest IH (See Supplemental Digital Content, Figure 2, http://links.lww.com/WNO/A53). A full spine MRI failed to disclose a cerebrospinal fluid leak. Lumbar puncture yielded normal spinal fluid but an opening pressure was not obtained owing to difficulty positioning the patient without causing discomfort. The epidural catheter was eventually removed. Follow-up examination 2 months later was normal. Case 6 A 35-year-old woman with Crohn disease underwent ileocecal revision with placement of a mid-thoracic epidural catheter for pain control. On the first postoperative day, she complained of severe headache exacerbated by sitting up. An epidural catheter was removed on the second post-operative day, but the headache worsened and she devel-oped neck discomfort, nausea, and vomiting. She was treated with narcotic analgesics. On the sixth postoperative day, she complained of intermittent diplopia (Table 1). Bedside examination disclosed normal visual acuity and confrontation visual fields, pupils, and funduscopy. She had full ocular ductions, but when fixating on a distant target, she noted diplopia in extremes of lateral gaze. On cover testing, the patient was orthotropic in primary position with 15 prism-diopters of esotropia in right and left gaze. The patient reported worsening headache with pain extending down her back, together with chills, raising concern for spinal epidural hematoma or abscess. Brain MRI showed grade 2 pachymeningeal enhancement and thickening, grade 2 enlargement of the pituitary gland, and grade 1 downward displacement of the brainstem (See Supplemental Digital Content, Figure 3, http://links.lww.com/WNO/A53). Spine MRI showed an extrathecal fluid collection thought to represent a leak. Neurological examination disclosed no evidence of spinal cord or root compression. On the ninth postoperative day, she underwent a lumbar blood patch procedure. The following day, headache resolved but diplopia persisted. Two weeks after surgery, the patient had full ocular ductions, with an 18 prism-diopter esotropia in primary and left gaze that increased to 25 prism-diopters on right gaze. Two months after the surgery, the diplopia had disappeared and ocular alignment was normal. DISCUSSION These 6 patients reported diplopia days to weeks after implantation of a spinal epidural catheter for postprocedural pain control (Cases 1, 2, 5, 6), postoperative fluid evacuation (Case 4), or following parturition (Case 3). Usually attributed to sixth nerve palsy, diplopia has been amply documented in IH (17-23). This report is distinctive in pointing out the confounding clinical features that delayed diagnosis for the following reasons: 1. Clinicians often did not realize that an epidural catheter had been in place. In some cases, the catheter had long been removed when the diplopia was first reported. When it was still in place, it often was not considered. Case 4 had a Jackson-Pratt epidural drain left in place postoperatively, a device about which clinicians caring for the patient were unfamiliar. 2. The diplopia was not reported immediately after the pro-cedure. The delay ranged from 5 to 14 days, such that clinicians did not consider it a consequence of the pro-cedure. In a comprehensive review of 95 cases of IH-associated sixth nerve palsy, the palsy was found to develop between 1 and 21 days after dural puncture, usually between 4 and 10 days (17). 3. The ocular motor findings were not obvious. The exam-ination was usually at the bedside. Because they were uncomfortable as they were recovering from their proce-dures, patients could not always cooperate adequately with ocular motility and alignment testing. Ductional FIG. 3. Case 3. A. Postcontrast T1 magnetic resonance imaging shows grade 1 pachymeningeal enhancement (arrows) and grade 2 enlargement of the pituitary gland (arrowhead). B. Postcontrast T1 sagittal image reveals grade 2 downward dis-placement of the brainstem and cerebellar tonsils (arrow). 110 Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. deficits were usually mild. In 2 patients (Cases 5, 6), bedside measurements suggested comitant esotropia attributable to a break in fusion, a common occurrence in postoperative patients treated with pain-relieving medications. But the comitant misalignment often per-sisted after the patients were on very low doses of nar-cotics that did not alter consciousness and would not have been expected to disrupt fusion. Accordingly, we believe that IH should be considered a cause, not merely of sixth nerve palsy, but also of comitant esodeviation with normal or near-normal ocular ductions. 4. A history of postural headache, so characteristic of IH, could not always be elicited. Some patients had no head-ache, perhaps because they were receiving pain medica-tion. Others were not ambulatory and were unable to report a postural component of their headache. In IH-associated sixth cranial nerve palsy, headache may pre-cede the palsy, follow it, or not occur at all (17). 5. There were clinical features that suggested a cause other than IH. Case 1 had a history of inflammatory bowel dis-ease and displayed conjunctival hyperemia, prompting con-sideration of an orbital process. Case 2 had weight loss and night sweats, leaving open the possibility of inflammatory or neoplastic meningitis. Case 3 had undergone evacuation of a subdural hematoma. Case 4 had metastatic melanoma. 6. Radiologists did not exclude a diagnosis of pachymeningi-tis. All 6 patients had diffuse pachymeningeal enhance-ment, the most common neuroimaging abnormality in IH (24), a sign attributed to dural vascular engorgement. Although a diffuse rather than a nodular pattern of pachy-meningeal enhancement is thought to favor IH over inflammatory or neoplastic pachymeningitis, the distinc-tion is imperfect, especially when the patient has clinical features that could be consistent with serious medical con-ditions (Cases 1, 2, 4) (25). Three patients (Cases 3, 4, 6) had signs of downward displacement of the brainstem, which should be definitive for IH, but the findings were subtle. Three patients had pituitary gland enlargement (Cases 2, 3, 6), a sign that, by itself, is not diagnostic of IH. One patient (Case 2) had subdural fluid collections, which, together with diffuse pachymeningeal enhance-ment, would strongly suggest IH. However, the collections were small and initially overlooked. Because the interpret-ing radiologists were unaware that an epidural pain cath-eter had been used, they were obligated to consider inflammatory or neoplastic causes of these imaging signs. Postdural puncture headache and diplopia are attributed to traction at the cranial base owing to downward displacement of the brainstem (17). PDPH usually subsides spontaneously within 1 week but can take up to 6 months (7,26,27). Epidural blood patch at the site of puncture, the preferred treatment for PDPH, is administered within 48 hours if the postural headache is not resolving. It was only performed in 2 of our patients (Cases 3, 6). Intracranial hypotension-associated diplopia is nearly always caused by sixth nerve palsy, believed to arise from traction at the petroclival junction, where the sixth cranial nerve is tethered in Dorello canal. The palsy is usually unilateral (80%) (17,20,21,23). Patients range in age from 17 to 69 years and men and women are affected equally. Spontaneous recovery occurs in 90% of patients within 8 months, the majority recovering fully within 3 months. If the palsy is present after 8 months, it will be permanent (17). Epidural blood patch, effective in relieving PDPH, does not alter the recovery of sixth nerve palsy (17). The consequences of not recognizing the connection between diplopia and IH are a fruitless and perhaps harmful diagnostic work-up that includes lumbar puncture, which in 2 of our patients (Cases 1, 5) led to "dry taps" and in 1 case (Case 4) to an unnecessary and risky cisternal punc-ture. In the experience of one of the authors (J.D.T.), repeated lumbar puncture in a patient with IH-associated diplopia may convert a partial into a total sixth nerve palsy without recovery. If an epidural pain control system has been used and one or more of the neuroimaging features of IH is present, a presumptive diagnosis of IH as the cause of diplopia can be justified without the need for lumbar puncture confirmation of low intracranial pressure. REFERENCES 1. Apfel CC, Saxena A, Cakmakkaya OS, Gaiser R, George E, Radke O. Prevention of postdural puncture headache after accidental dural puncture: a quantitative systematic review. Br J Anaesth. 2010;105:255-263. 2. Berger CW, Crosby ET, Grodecki W. North American survey of the management of dural puncture occurring during labour epidural analgesia. Can J Anaesth. 1998;45:110-114. 3. Gleeson CM, Reynolds F. Accidental dural puncture rates in UK obstetric practice. Int J Obstet Anesth. 1998;7:242-246. 4. Sprigge JS, Harper SJ. Accidental dural puncture and post dural puncture headache in obstetric anaesthesia: presentation and management: a 23-year survey in a district general hospital. Anaesthesia. 2008;63:36-43. 5. Scavone BM, Wong CA, Sullivan JT, Yaghmour E, Sherwani SS, McCarthy RJ. Efficacy of a prophylactic epidural blood patch in preventing post dural puncture headache in parturients after inadvertent dural puncture. Anesthesiology. 2004;101: 1422-1427. 6. Cesur M, Alici HA, Erdem AF, Silbir F, Celik M. Decreased incidence of headache after unintentional dural puncture in patients with cesarean delivery administered with postoperative epidural analgesia. J Anesth. 2009;23:31-35. 7. Bezov D, Ashina S, Lipton R. Post-dural puncture headache: part II-prevention, management, and prognosis. Headache. 2010;50:1482-1498. 8. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286-2296. 9. Mokri B, Krueger BR, Miller GM, Piepgras DG. Meningeal gadolinium enhancement in low-pressure headaches. J Neuroimaging. 1993;3:11. 10. Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 2006;67:1088-1089. 11. Chung SJ, Kim JS, Lee MC. Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology. 2000;55:1321-1327. 12. Atkinson JL, Weinshenker BG, Miller GM, Piepgras DG, Mokri B. Acquired Chiari I malformation secondary to spontaneous spinal Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 111 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. cerebrospinal fluid leakage and chronic intracranial hypotension syndrome in seven cases. J Neurosurg. 1998;88:237. 13. Bakshi R, Mechtler LL, Kamran S, Gosy E, Bates VE, Kinkel PR, Kinkel WR. MRI findings in lumbar puncture headache syndrome: abnormal dural-meningeal and dural venous sinus enhancement. Clin Imaging. 1999;23:73-76. 14. Mokri B, Atkinson JL. False pituitary tumor in CSF leaks. Neurology. 2000;55:573-575. 15. Alvarez-Linera J, Escribano J, Benito-León J, Porta-Etessam J, Rovira A. Pituitary enlargement in patients with intracranial hypotension syndrome. Neurology. 2000;55:1895-1897. 16. Farb RI, Forghani R, Lee SK, Mikulis DJ, Agid R. The venous distension sign: a diagnostic sign of intracranial hypotension at MR imaging of the brain. AJNR Am J Neuroradiol. 2007;28:1489-1499. 17. Nishio I, Williams BA, Williams JP. Diplopia: a complication of dural puncture. Anesthesiology. 2004;100:158-164. 18. Yatziv Y, Stolowitch C, Segev Y, Kesler A. Acute esotropia after epidural anesthesia. Obstet Gynecol. 2008;111:540-541. 19. Kose KC, Cebesoy O, Karadeniz E, Bilgin S. Eye problem following foot surgery-abducens palsy as a complication of spinal anesthesia. Med GenMed. 2005;7:15. 20. Schober P, Loer SA, Schwarte LA. Paresis of cranial nerve VI (N. abducens) after thoracic dural perforation. Minerva Anestesiol. 2010;76:1085-1087. 21. Corbonnois G, O'Neill T, Brabis-Henner A, Schmitt E, Hubert I, Bouaziz G. Unrecognized dural puncture during epidural analgesia in obstetrics later confirmed by brain imaging. Ann Fr Anesth Reanim. 2010;29:584-588. 22. Arcand G, Girard F, McCormack M, Chouinard P, Boudreault D, Williams S. Bilateral sixth cranial nerve palsy after unintentional dural puncture. Can J Anaesth. 2004;51:821-823. 23. Khemka S, Mearza AA. Isolated sixth nerve palsy secondary to spontaneous intracranial hypotension. Eur J Neurol. 2006;13:1264-1265. 24. Su CS, Lan MY, Chang YY, Lin WC, Liu KT. Clinical features, neuroimaging and treatment of spontaneous intracranial hypotension and magnetic resonance imaging evidence of blind epidural blood patch. Eur Neurol. 2009;61:301-307. 25. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525-551. 26. Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718-729. 27. Dripps RD, Vandam LD. Long-term follow-up of patients who received 10,098 spinal anesthetics: failure to discover major neurological sequelae. J Am Med Assoc. 1954;156: 1486-1491. 112 Sudhakar et al: J Neuro-Ophthalmol 2013; 33: 106-112 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |