| OCR Text |
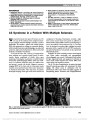
Show Distinguishing Optic Neuritis in Neuromyelitis Optica Spectrum Disease From Multiple Sclerosis: A Novel Magnetic Resonance Imaging Scoring System Mithu Storoni, MA(Cantab), MRCOphth, Indran Davagnanam, FRCR, Mark Radon, MA(Cantab), FRCR, Ata Siddiqui, FRCR, Gordon T. Plant, MD, FRCP, FRCOphth Background: The management of acute optic neuritis differs according to the underlying etiology and techniques which may help with early differential diagnosis are there-fore of considerable value. Objective: We wanted to determine if multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) could be differentiated on the basis of neuroimaging abnormalities of the anterior visual pathways following an episode of optic neuritis. Methods: Magnetic resonance imaging (MRI) findings of 27 patients diagnosed with MS (n = 15) or NMOSD (n = 12), who presented with acute isolated optic neuritis over a 3-year period, were reviewed retrospectively. The extent and location of inflammation along the anterior visual path-ways were analyzed. A novel scoring system was devised, based upon the number of anatomical segments involved. Results: Patients with NMOSD had a relative risk of 7.5 (confidence interval: 0.3-17.3) of having a score of 4 or more. Only NMOSD patients were found to have a score of 6 or higher. A trend for more posterior involvement of the anterior visual pathways was noted in the NMOSD group. Conclusion: This pilot study suggests that the MRI-based scoring system described here may aid in distinguishing patients with optic neuritis who have MS vs NMOSD. Visual pathway inflammation in NMOSD patients appears to be more extensive than in MS, mirroring the longitudi-nally extensive spinal cord lesions found in neuromyelitis optica. Journal of Neuro-Ophthalmology 2013;33:123-127 doi: 10.1097/WNO.0b013e318283c3ed © 2013 by North American Neuro-Ophthalmology Society Acute isolated optic neuritis may be the first manifestation of both multiple sclerosis (MS) and neuromyelitis optica (NMO). The discovery of the aquaporin 4 autoantibody (AQP4-Ab) has provided serological markers to distinguish NMO from MS and led to the description of neuromyelitis optica spectrum disorder (NMOSD) (1,2) (Table 1). Patients with NMO may experience a long temporal delay after acute optic neuritis before a relapse in the form of transverse myelitis occurs (2). In such cases, an episode of optic neuritis caused by NMO may be indistinguishable clinically from optic neuritis caused by MS. Comparison of brain mag-netic resonance imaging (MRI) findings may be limited as Matsushita et al (3) have shown in patients who are seropos-itive for AQP4-Ab and those with typical MS. However, would MRI of the anterior visual pathways be more useful in distinguishing patients with NMO from MS? Khanna et al (4) have reported a trend for more posterior lesions within the anterior visual pathways in patients with NMO and chiasmatic involvement occurring only in NMO. They found no significant difference in the length of the inflammatory lesion between the 2 groups. In this pilot study, we compared the MRI appearance of the anterior visual pathways in acute optic neuritis in NMOSD to MS. We devised a simple scoring system to evaluate 2 aspects of the MR abnormalities: the linear location and thickness of the cross-sectional area (CSA). Departments of Neuro-Ophthalmology (GTP, MS) and Radiology (ID), Moorfields Eye Hospital, London, United Kingdom; Department of Neuro-Ophthalmology, The National Hospital for Neurology and Neurosurgery (MS, ID, MR, GTP), London, United Kingdom; and the Medical Eye Unit (GTP) and the Department of Radiology (AS), St. Thomas' Hospital, London, United Kingdom. Supported by the UCLH/UCL Comprehensive Biomedical Research Centre. M. Storoni was supported by a Fight for Sight Clinical Research Fellowship award. Preliminary observations for this study were reported at the 2011 European Neuro-Ophthalmology Society Meeting in Barcelona, Spain, and the findings of this study were presented at the 2012 North American Neuro-Ophthalmology Society Meeting in San Antonio, TX. Disclosures: The authors report no conflicts of interest. Address correspondence to Gordon T. Plant, MD (Cantab), FRCP, FRCOPhth, Department of Neuro-Ophthalmology, The National Hos-pital for Neurology and Neurosurgery, Box 93, Queen Square, London WC1N 3BG, United Kingdom; E-mail: gordon@plant.globalnet.co.uk Storoni et al: J Neuro-Ophthalmol 2013; 33: 123-127 123 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. METHODS This was a retrospective pilot study in which the MRI results of 27 patients were studied. Fifteen patients had confirmed MS and 12 patients had NMOSD. All patients presented over a 3-year period with acute isolated optic neuritis and were scanned using a 1.5-tesla or 3.0-tesla scanner during the acute phase (all within 6 weeks of symptom onset). Patients with coexisting neurological or systemic illness causing other visual pathway or brain lesions were excluded. A diagnosis of NMOSD was given to patients who met established diagnostic criteria (2) (Table 1). Multiple sclerosis was diagnosed according to the revised McDonald criteria (5). All patients were tested for AQP4-Ab. Testing was carried out at theWetherall Institute ofMolecular Medicine, University of Oxford, by a method using the fluorescence immunoprecipi-tation assay technique described elsewhere (6). Multiple scle-rosis patients were all seronegative for AQP4-Ab. Neuroimaging The majority of patients had MRI of the anterior visual pathways using standardized clinical protocols, performed on a General Electric Discovery MR450 1.5-tesla MRI unit (GE Healthcare, Waukesha, WI) or Siemens Trio 3-tesla MRI unit (Siemens AG, Erlangen, Germany). As data was collected over several years, some examinations were acquired with other scanners with minor variations in acquisition protocols. All MRIs included coronal T2 fat-suppressed and T1 images of the anterior visual pathways in addition to imaging of the brain and/or spine. Intravenous contrast was used in selected cases. Imaging parameters for the coronal T2 fat-suppressed sequences were 1) General Electric Discovery MR450 1.5- tesla MRI: fat saturation, echo delay time (TE) 102.0, repetition time (TR) 4983.0, sample averaging (NEX) 3, base resolution 384, field of view (FoV) 18.0, slice thickness 3.0 mm and 2) Siemens Trio 3-tesla MRI: fat saturation, TE 84.0, TR 5020.0, averages 3, base resolution 384, FoV 18.0, slice thickness 2.0 mm. Imaging parameters for the coronal T1 sequences were 1) General Electric Discovery MR450 1.5-tesla MRI: TE 8.0, TR 597.0, NEX 4, base resolution 256, FoV 18.0, slice thickness 3.0 mm and 2) Siemens Trio 3-tesla MRI: fat saturation, TE 8.2, TR 500.0, averages 2, base resolution 256, FoV 18.0, slice thickness 2.0 mm. Magnetic resonance images were assessed independently by 2 neuroradiologists (I.D. and M.R.), who were blinded to the patients' history and diagnosis. A consensus decision was reached in case of disagreement. Protocol for the Presence of Inflammation Contrast-enhanced MRI has been reported as the gold standard for the detection of inflammation in the visual pathways (7). In accordance with local hospital protocol, gadolinium was not used in the majority of our cases. Increase in the thickness or CSA of the affected part of the anterior visual pathways occurring during acute optic neuritis was used as an absolute marker for inflammation (8). The presence of optic nerve T2 signal hyperintensity supported the presence of inflammation; it was not considered an abso-lute marker for inflammation as its persistence following the resolution of acute optic neuritis has been reported (8). TABLE 1. Features of neuromyelitis optica spectrum disorder NMO Limited forms of NMO Single or recurrent events of longitudinally extensive myelitis ($3 vertebral segment spinal cord lesion detected on MRI) Optic neuritis: recurrent or simultaneous bilateral Asian optic-spinal MS Optic neuritis or longitudinally extensive myelitis associated with systemic autoimmune disease Optic neuritis or myelitis associated with brain lesions typical of NMO (hypothalamic, corpus callosal, periventricular, or brainstem) MRI, magnetic resonance imaging; MS, multiple sclerosis; NMO, neuromyelitis optica. Adapted from Wingerchuk et al (2). FIG. 1. Schematic representation of the anterior visual pathways divided into 10 segments. 124 Storoni et al: J Neuro-Ophthalmol 2013; 33: 123-127 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. Image Analysis Anterior visual pathways were divided into 10 segments: orbital, canalicular, and intracranial segments of the left and right optic nerves, the left and right halves of the optic chiasm, and the left and right optic tracts (Fig. 1). T2 fat-suppressed and corresponding T1 sequences were used to assess CSA and T2 signal hyperintensity. The number of anatomical segments affected by an increase in CSA at any point on the segment was noted in each case. A score of +1 was given for each affected segment, such that FIG. 2. Pattern of visual pathway involvement in the neuromyelitis spectrum disease (NMOSD) group (n = 12). Each affected segment is represented by a solid line, and unaffected segments are represented by a dotted line. FIG. 3. Pattern of visual pathway involvement in the multiple sclerosis (MS) group (n = 15). Each affected segment is represented by a solid line, and unaffected segments are represented by a dotted line. Storoni et al: J Neuro-Ophthalmol 2013; 33: 123-127 125 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. a patient with the involvement of all segments would be given a score of 10. A segment was not required to be thickened along its entire length in order for it to be given a score of +1. The entire anatomical extent of the lesion did not need to be continuous along the extent of positive scoring. Statistical Analysis Lesion extent scores were compared between MS and NMOSD groups using the Mann-Whitney rank sum test. A P value of 5% was used to define statistical significance. The relative risk (RR) of higher scoring was calculated for the 2 groups. The involvement of each segment was compared between the 2 groups using the 2-tailed Fisher exact test. A P value of 5% was used to define statistical significance. The RR of the involvement of each segment for the 2 groups was calculated. RESULTS Twelve patients were diagnosed with NMOSD and 15 patients with MS. The female to male ratio was 10:2 in the NMOSD group and 11:4 in the MS group. The mean age of patients was 39 years (range 27-51) and 34 years (range 26-42) in the NMOSD group and MS group, respectively. Caucasians comprised 69% of the MS patients and 17% of those with NMOSD. Figures 2 and 3 are schematic illus-trations demonstrating the pattern of visual pathway involvement in the NMOSD and MS groups. Lesion Extent Figure 4 shows the lesion extent scores in optic neuritis patients with NMOSD and MS. Patients with MS demon-strated a mean score of 2.2 (range, 1-5) compared with a mean score of 4.0 (range, 2-7) in NMOSD patients. The difference between the means was statistically signifi-cant (P = 0.007). The RR of having a lesion extent score 4 in NMOSD vs MS was 7.5 (95% confidence interval: 0.33-17.3). A score of greater than 6 was seen only in patients with NMOSD. Lesion Site Table 2 shows the frequency of involvement of each site and the RR for NMOSD over MS at each site across the patients within each group. A trend for anterior involvement was seen in MS patients. The RR of segment involvement within the NMOSD group increased with a more posterior location (RR for optic tract involvement = 3.13 vs RR for intracana-licular involvement = 1.25). The number of NMOSD patients with chiasmal involvement was significantly greater than the number of MS patients (P = 0.021). Both MS (n = 2) and NMOSD (n = 5) patients displayed bilateral optic chiasmal involvement. DISCUSSION Our study demonstrates that a novel MRI-based scoring system may help differentiate optic neuritis in patients with NMOSD vs MS. A lesion extent score $4 is highly sug-gestive of NMOSD. Anterior visual pathway inflammation in optic neuritis secondary to NMOSD may mirror the longitudinally extensive spinal cord lesions found in NMO. While lesion distribution was not demonstrably different between NMO and MS patients, predilection was found for FIG. 4. Distribution of lesion extent scores in patients with neuromyelitis spectrum disease (NMOSD) and multiple sclerosis (MS)-associated optic neuritis. Mean values and 5% and 95% percentiles are shown. The difference between the means was statistically significant (P = 0.007). TABLE 2. The frequency of involvement and relative risk of neuromyelitis spectrum disease and multiple sclerosis at each affected site Location NMO (n = 12), % MS (n = 15), % P Relative Risk* Orbital 5 (42) 10 (67) 0.258 0.63 (0.38-1.74) Canalicular 10 (83) 10 (67) 0.408 1.25 (0.71-1.71) Cranial 9 (75) 5 (33) 0.054 2.25 (0.65-3.12) Chiasm 9 (75) 4 (27) 0.021* 2.81 (0.64-3.86) Optic tract 5 (42) 2 (13) 0.185 3.13 (0.38-7.02) P values were calculated using the 2-tailed Fisher exact test. *Ninety-five percent confidence intervals are provided in parentheses. MS, multiple sclerosis; NMO, neuromyelitis optica. 126 Storoni et al: J Neuro-Ophthalmol 2013; 33: 123-127 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. more posterior segments in NMOSD patients and for more anterior segments in MS. This is consistent with previous reports (4,9). Chiasmal inflammation was more frequent in patients with NMOSD than MS. This is in contrast to the findings of Khanna et al (4), where chiasmal involvement was found exclusively in NMO patients. In that study, the use of smaller sample sizes (NMO: n = 6; MS: n = 11) and differing imaging techniques (exclusive use of 1.5-tesla magnet) may explain these differences. Our study has a number of limitations including small number of patients and the lack of use of intravenous contrast. The presence of increased CSA as the criterion for assessing the presence of inflammation along the visual pathway may have excluded patients with prior optic atrophy. As this was a pilot study, there was no standard protocol for the time interval between onset of optic neuritis and the time of scanning or examination of visual parameters. Although a trend for more extensive visual pathway inflammation was observed in NMOSD, the degree of inflammation may have been under-estimated as corticosteroid therapy was sometimes initiated on patients with NMOSD prior to MRI. In conclusion, the results of this study suggest that a scoring system based on the findings of MRI of the anterior visual pathways may help to identify the etiology of acute optic neuritis. This has important clinical implications given the differences in evaluation and treatment of patients with NMOSD vs MS. REFERENCES 1. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Pittock SJ, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106-2112. 2. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805-815. 3. Matsushita T, Isobe N, Piao H, Matsuoka T, Ishizu T, Doi H, Masaki K, Yoshiura T, Yamasaki R, Ohyagi Y, Kira J. Reappraisal of brain MRI features in patients with multiple sclerosis and neuromyelitis optica according to anti-aquaporin-4 antibody status. J Neurol Sci. 2010;291:37-43. 4. Khanna S, Sharma A, Huecker J, Gordon M, Naismith RT, Van Stavern GP. Magnetic resonance imaging of optic neuritis in patients with neuromyelitis optica versus multiple sclerosis. J Neuroophthalmol. 2012;32:216-220. 5. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292-302. 6. Waters P, Jarius S, Littleton E, Leite MI, Jacob S, Gray B, Geraldes R, Vale T, Jacob A, Palace J, Maxwell S, Beeson D, Vincent A. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913-919. 7. Youl BD, Turano G, Miller DH, Towell AD, MacManus DG, Moore SG, Jones SJ, Barrett G, Kendall BE, Moseley IF, et al. The pathophysiology of acute optic neuritis. An association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114:2437-2450. 8. Youl BD, Turano G, Towell AD, Barrett G, MacManus DG, Moore SG, Miller DH, Jones SJ, du Boulay EP, Kendall BE, Moseley IF, McDonald WI. Optic neuritis: swelling and atrophy. Electroencephalogr Clin Neurophysiol Suppl. 1996;46:173- 179. 9. Zou X, Pang Y, Li X, Zhang Y, Li M, Liang C, Huang B, Huang M. Magnetic resonance imaging in 40 cases of optic neuritis [in Chinese]. Zhonghua Yan Ke Za Zhi. 1999;35:422-425. Storoni et al: J Neuro-Ophthalmol 2013; 33: 123-127 127 Original Contribution Copyright © North American Neuro-Ophthalmology Society. Unauthorized reproduction of this article is prohibited. |