| Title |
Magnetic Resonance Imaging of Suspected Cervicocranial Arterial Dissections |
| Creator |
Shah, GV; Quint, DJ; Trobe, JD |
| Affiliation |
Department of Radiology (Neuroradiology), University of Michigan Medical Center, Ann Arbor, Michigan 48105, USA. |
| Abstract |
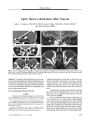
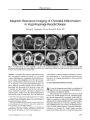
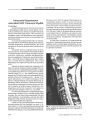
The authors propose that the optimal screening protocol for evaluation of suspected cervicocranial arterial dissections is magnetic resonance imaging (MRI) that includes three components: 1) contrast-enhanced three-dimensional time-of-flight magnetic resonance angiography (MRA) through the superior mediastinum, neck, and skull base; 2) three-dimensional multiple overlapping thin-section acquisition MRA of the skull base and Circle of Willis region; and 3) axial non-contrast, non-fat-suppressed T1-weighted, fat-suppressed T1-weighted, and T2-weighted spin-echo MRI from the level of the aortic arch through the level of the circle of Willis. MRA permits visualization of vascular luminal narrowing or obliteration, which can suggest vascular dissection but can also be caused by congenital variation, dysplasia, intraluminal thrombus, vasospasm, or extramural compression by tumor. By directly visualizing the blood vessel wall, axial T1-weighted and T2-weighted spin-echo MRI can identify the intramural hemorrhage of vascular dissection. This protocol is designed to maximize the sensitivity of a noninvasive technique and may eliminate the need for conventional endovascular angiography. |
| Subject |
Adult; Brain Infarction/pathology; Carotid Artery Diseases/diagnosis; Humans; Image Processing, Computer-Assisted/methods; Imaging, Three-Dimensional/methods; Magnetic Resonance Imaging/methods; Male; Spinal Cord/pathology; Tomography, X-Ray Computed/methods; Vertebral Artery/pathology; Vertebral Artery Dissection/diagnosis |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225336 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6xq0b1q/225336 |