| Title |
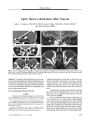
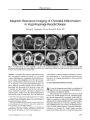
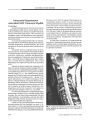
Utility of Source Images of Three-Dimensional Time-of-Flight Magnetic Resonance Angiography in Indirect Carotid-Cavernous Sinus Fistulas |
| Creator |
Tsai, YF; Chen, LK; Su, CT; Lu, TN; Wu, CC; Kuo, CJ |
| Affiliation |
Department of Diagnostic Radiology, Shin Kong Wu Ho-Su Memorial Hospital, Shih Lin, Taipei, Taiwan. yuhfeng.tsai@msa.hinet.net |
| Abstract |
BACKGROUND: We sought to assess the relative contribution of magnetic resonance imaging (MRI), maximum intensity projection (MIP), and source images of three-dimensional (3D) time-of-flight (TOF) magnetic resonance angiography (MRA) to the diagnosis of indirect (dural) carotid-cavernous sinus fistulas (CCFs). METHODS: MRI and 3D TOF MRA were obtained in eight consecutive patients with indirect CCFs confirmed by conventional catheter angiography. Two radiologists masked to the angiographic results reviewed images retrospectively to evaluate the efficacy of MRI and 3D TOF MRA source and MIP images in the diagnosis of CCF. RESULTS: MRI disclosed CCF in five of eight cases; MIP images of TOF MRA disclosed CCF in four cases; source images of TOF MRA disclosed all eight CCF cases. CONCLUSIONS: The MRA source images are indispensable for a confirmatory diagnosis of indirect (dural) CCF. Underdiagnosis may occur by relying on MRI or 3D TOF MIP images alone. |
| Subject |
Adult; Older people; Older people, 80 and over; Carotid-Cavernous Sinus Fistula/pathology; Cerebral Angiography/methods; Female; Humans; Imaging, Three-Dimensional/methods; Magnetic Resonance Angiography/methods; Male; Middle Older people; Retrospective Studies; Sensitivity and Specificity |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
225329 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6xq0b1q/225329 |