| Title |
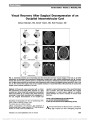
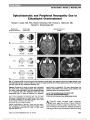
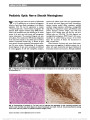
A 16-Year-Old Boy With a Suprasellar Mass |
| Creator |
Tamhankar, Madhura A; Paessler, Michele E; Kharlip, Julia N; Shekdar, Karuna V; Burnham, Jon M; Cole, Kristina A; Volpe, Nicholas J; Lee, Andrew G |
| Affiliation |
Department of Ophthalmology (MAT), Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania; Department of Endocrinology (JNK), University of Pennsylvania, Philadelphia, Pennsylvania; Departments of Pathology (MEP), Radiology (KVS), Rheumatology (JMB), and Oncology (KAC), Children's Hospital of Philadelphia, University of Pennsylvania, Philadelphia, Pennsylvania |
| Subject |
Adolescent; Brain Neoplasms; Central Nervous System Cysts; Diagnostic Techniques, Ophthalmological; Humans; Magnetic Resonance Imaging; Male; Neurologic Examination; Proto-Oncogene Proteins c-kit |
| Format |
application/pdf |
| Publication Type |
Journal Article |
| Collection |
Neuro-Ophthalmology Virtual Education Library: Journal of Neuro-Ophthalmology Archives: https://novel.utah.edu/jno/ |
| Publisher |
Lippincott, Williams & Wilkins |
| Holding Institution |
Spencer S. Eccles Health Sciences Library, University of Utah |
| Rights Management |
© North American Neuro-Ophthalmology Society |
| Setname |
ehsl_novel_jno |
| ID |
227620 |
| Reference URL |
https://collections.lib.utah.edu/ark:/87278/s6kh3tdd/227620 |